Tags
Business, Center for Devices and Radiological Health, Consulting, DePuy, Federal Food Drug and Cosmetic Act, Food & Drug Administration, Hip Replacement, Institute of Medicine, Medical device, Medicine, New England Journal of Medicine
Recall Index – Second Quarter 2011 – Medical Devices
Source:expertrecall.com
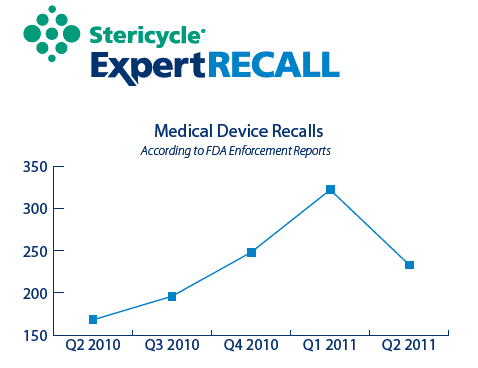
During the second quarter of 2011, FDA Enforcement Reports documented 233 medical device recalls involving nearly 16 million units. This reflects a 27 percent decrease in recalls over the previous quarter, but a 38 percent increase in the number of recalls over tile same quarter last year. The first quarter of 2011 saw 322 recalls involving more than 43 million units. There were ten recalls in the second quarter that involved more than 100,000 units.
Medical device recalls documented during the second quarter of 2011 affected consumers in the U.S. and overseas. More than 80 percent of recalls affected consumers in the U.S. Nearly 170 recalls affected both domestic and international consumers. Three recalls affected only international consumers during the second quarter.
The ExpertRECALL Index also revealed the following:
- Medical device recalls in the second quarter of 2011 declined for the first time in the past five quarters.
- Second quarter recalls affected the fewest units in the past five quarters.
- More than 30 companies initiated more than one recall in the second quarter.
- Only four percent of recalls in the second quarter of 2011 were classified as Class I.
- Ninety percent of medical device recalls in the second quarter were classified as Class II recalls, accounting for almost 98 percent of all units

Related articles
- IOM Gives FDA The wrong prescription on medical devices & 510(k) (earlsview.com)
- Report: FDA’s Medical Device Approval Process Is Flawed (earlsview.com)
- US Congress Women Rosa L. DeLauro Takes FDA To Task Over Comments re IOM Report (earlsview.com)
- Report could sway FDA device review process (earlsview.com)
- Expert group calls for new way to clear medical devices (earlsview.com)
- Australian TGA Response to Recall of DePuy ASR Hip Replacement (earlsview.com)
- U.S. advisers call for new medical device regime (earlsview.com)
- Bruce Greenfield may be the last New Zealander implanted with a faulty hip joint (earlsview.com)
- Medical device management often underestimated (medcitynews.com)
- Regulatory Reform Series #5 – FDA Medical Device Regulation: Impact on American Patients, Innovation and Jobs (earlsview.com)

Pingback: Sadwin gets to the heart of the Issues with FDA 510(k) « Earl's View
Pingback: FDA Loosens Premarket Notification Requirements for 30 Medical Device Types « Earl's View
Pingback: Medical Device Aproval – Divisive Devices or UFC 510(k): FDA Meets IOM « Earl's View
Pingback: FDA Proposes Guidelines That Clarify Benefit-Risk Determinations For Medical Devices « Earl's View