Tags
chrome poisoning, Cobalt chrome, Cobalt poisoning, DePuy, DePuy ASR Hip, depuy hip recall, DePuy Hip Recall Litigation, DePuy Lawsuit, DePuy Pinnacle Hip Replacement System, Hip failure, Hip recall, Hip Replacement, hip resurfacing, hip revision, Johnson & Johnson, metal ions, metal-on-metal hips, metallosis, MoM Hips
DePuy Pinnacle Hip Implant Named in Lawsuits, Calls for Recall Grow
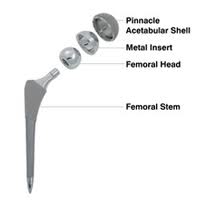
DePuy Orthopaedics, the Johnson & Johnson medical device division that last year recalled ASR XL Acetabular System and the ASR Hip Resurfacing System, is facing legal woes over another hip implant. A growing number of lawsuits involving the DePuy Pinnacle hip implant have been filed in recent months, many of them alleging problems that will sound familiar to anyone who has been following DePuy ASR hip implant recalls. Many of these lawsuits contend that the DePuy Pinnacle should have been subject to a recall, much like the one DePuy issued for its ASR hip implant devices last August.

The DePuy Pinnacle device is, like the ASR devices, a metal-on-metal hip implant. According to DePuy Some Pinnacle hip implant lawsuits, the device may shed small metal particles as its components rub against each other. This can cause tissue breakdown, bone loss, and even the formation of non-cancerous tumors. The shedding of metal shavings can cause cobalt poisoning, a disorder that, if left untreated, can put patients at risk of tinnitus (ringing in the ears), vertigo, deafness, blindness, optic nerve atrophy, convulsions, headaches, peripheral neuropathy, cardiomyopathy, and hypothyroidism. In some cases, patients with metal-on-metal hip implants may develop an inflammatory reaction, known as metallosis, to these metal particles.
According to one DePuy Pinnacle hip implant lawsuit, filed in early December in U.S. District Court for the Western District of Washington, the plaintiff has undergone six surgeries due to metal poisoning he sustained as a result of a Pinnacle hip implant he received in 2007.A second suit filed in November U.S. District Court for the Central District of California alleges that another Pinnacle recipient has suffered abnormal gait, nerve pain and other problems since receiving the device in 2004. That plaintiff is soon to undergo revision surgery.
Most recently, a Tennessee man filed suit over a DePuy Pinnacle hip implant he received in May 2009. According to that lawsuit, the plaintiff was forced to undergo revision surgery less than one year later, after the acetabular component of the left hip loosened due to friction and natural biologic corrosion. Even though his implant was replaced, the plaintiff is still unable to walk without the use of a cane.

Over the past year, concerns over the safety of metal-on-metal hip implants, such as the DePuy Pinnacle and ASR devices, have continued to grow. According to a New York Times report published in March 2010, studies in recent years indicate that in some cases the devices can quickly begin to wear, generating high volumes of metallic debris that is absorbed into a patient’s body, leading to cobalt poisoning. The limited studies conducted so far on metal-on-metal hip implants estimate that 1 to 3 percent of implant recipients could be affected by such problems.
In October, the American Academy of Orthopaedic Surgeons issued a warning about the devices, while the U.S. Food & Drug Administration (FDA) recently launched a website to provide information about metal-on-metal hip implants and their potential problems.
