Tags
American Academy of Orthopaedic Surgeons, chrome poisoning, Cobalt chrome, Cobalt Ions, Cobalt poisoning, DePuy, depuy hip recall, DePuy Hip Recall Litigation, DePuy Lawsuit, DePuy Pinnacle Hip Replacement System, FDA Hip Recall, FDA Safety Study, Food & Drug Administration, Hip failure, Hip recall, Hip Replacement, Johnson & Johnson, lawsuit, Metal Hypersensitity, metal ions, metal-on-metal hips, metallosis, MoM Hips, Multidistrict litigation, osteoarthritis, Total Hip Replacement
MDL Sought for Growing Number of DePuy Pinnacle Hip Implant Lawsuits

Another lawsuit has been filed against DePuy Orthopaedics involving its metal-on-metal Pinnacle hip implant. This latest DePuy Pinnacle hip replacement lawsuit, which was filed in the U.S. District Court for the Eastern District of Louisiana, involves five Pinnacle implant recipients. In a separate development, another DePuy Pinnacle hip implant victim is seeking to have the growing litigation surrounding the device consolidated in a single federal court.
All of the plaintiffs in the latest lawsuit – three men and two women – claim the DePuy Pinnacle hip implant failed prematurely, and resulted in elevated levels of cobalt in the blood, permanent disability, severe and prolonged pain, and other complications. The lawsuit also alleges that the DePuy Pinnacle hip implant is substantially equivalent to the defective DePuy ASR hip implant, which was recalled last August. Since the ASR recall was announced, more than 1,300 people have filed an adverse event report with the U.S. Food & Drug Administration (FDA) involving problems with a DePuy Pinnacle hip.
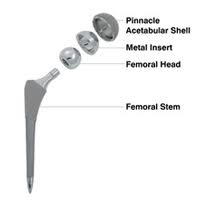
The DePuy Pinnacle device is, like the ASR hip replacement, a metal-on-metal hip implant. Concerns have been growing for some time that such devices may shed small metal particles as its components rub against each other. This can cause tissue breakdown, bone loss, and even the formation of non-cancerous tumors. The shedding of metal shavings can cause cobalt poisoning, a disorder that, if left untreated, can put patients at risk of tinnitus (ringing in the ears), vertigo, deafness, blindness, optic nerve atrophy, convulsions, headaches, peripheral neuropathy, cardiomyopathy, and hypothyroidism. In some cases, patients with metal-on-metal hip implants may develop an inflammatory reaction, known as metallosis, to these metal particles.
In October, the American Academy of Orthopaedic Surgeons issued a warning about the devices, while the FDA recently launched a website to provide information about metal-on-metal hip implants and their potential problems.
It is very possible that the litigation surrounding the DePuy Pinnacle device could become as large as that involving the ASR hip implant. Recently, a motion was filed with the U.S. Judicial Panel on Multidistrict Litigation to consolidate all pending and future federal DePuy Pinnacle hip implant lawsuits in a multidistrict litigation (MDL). The plaintiff who made the motion asked the panel to consolidate the Pinnacle hip implant lawsuits in a their own MDL, or make them part of the DePuy ASR hip implant MDL currently underway in the U.S. District Court for the Northern District of Ohio. The panel will hear oral arguments on the matter next month.
An MDL allows lawsuits associated with a particular product to be coordinated under one judge for pretrial litigation to avoid duplicative discovery, inconsistent rulings and to conserve the resources of the parties, witnesses and the court. When lawsuits are consolidated as a multidistrict litigation, each retains its own identity. If the multidistrict litigation process does not resolve the cases, they are transferred back to the court where they originated for trial.
Technorati Tags: Chrome Poisoning , Cobalt Chrome, Cobalt Ions, Cobalt Poisoning, DePuy, DePuy Hip Recall, DePuy Hip Recall Litigation, DePuy Lawsuit, DePuy Pinnacle Hip Replacement System, FDA Hip Recall, FDA Safety Study, Hip failure, Hip recall, Johnson & Johnson, Metal Hypersensitity, Metal Ions, Metal on Metal Hips, Metallosis, MoM hips, Osteoarthritis, Total Hip Replacement
Related articles
- DePuy Pinnacle Implants May Cause Same Problems as Defective ASR Implants (earlsview.com)
- DePuy Hip Recall Takes Toll on Johnson & Johnson Finances (earlsview.com)
- Will the DePuy Pinnacle Bring the Next Wave of Lawsuits? (earlsview.com)
- Crucial Documents Could Determine DePuy Hip Replacement Lawsuit Results (earlsview.com)
- Veterans are Put through Another War – Hip Recall (earlsview.com)
