Tags
Cobalt chrome, Cobalt poisoning, DePuy, DePuy ASR™ Hip Resurfacing System, depuy hip recall, depuy litigation, DePuy Orthopaedics, DePuy Pinnacle System, Food & Drug Administration, hip, Hip Replacement, Johnson & Johnson, metal, Total Hip Replacement, UNited States
Johnson & Johnson – DePuy Ceramic-on-Metal Total Hip Replacement System Approved
Based on an original post by Mark Crane (Source) Download here which was noted in my post here – click here. On June 14, 2011, the US Food and Drug Administration (FDA) approved “he first ceramic-on-metal total artificial hip system for patients with osteoarthritis.

DePuy CoMplete System
The Pinnacle CoMplete Acetabular Hip System (DePuy Orthopaedics Inc) is the first to combine a ceramic ball and a metal socket. Yesterday’s approval was based on a 2-year, randomized clinical trial that found no clinical difference between 194 patients who received the new ceramic-on-metal system and 196 patients in a control group who received a metal-on-metal hip implant. Two patients who received the ceramic-on-metal system required a second surgery to replace their new implant compared with 3 patients who required a second surgery in the control group As a condition of approval, the manufacturer will conduct a post-marketing study, monitoring patients receiving the new system for adverse events and metal ion concentrations in their blood.
My View
What were those famous words – “be afraid, be very afraid”….
So here we have the guys who have been single-handedly responsible for what looks to be one of the biggest medical disasters in recent times, causing potentially hundreds of thousands of patients all around the world huge medical problems, pain and suffering (plus other companies helping too)….
Look at this DePuy Attachment for the new Pinnacle CoMplete Acetabular Hip System – click here…
Some really fascinating stuff here – correct me if I am wrong…
- This is ceramic on metal – ceramic particles are great at grinding…
- The acetablular cup is cobalt chrome molybdenum…
- They also have a metal on metal version – with a cobalt chrome molybdenum femoral head…
- The femoral stem is cobalt chrome molybdenum…
- Cobalt “always” leaches out of hip implants…
- This device has just been approved based on 2 years study and about 400 patients.
- “the manufacturer will conduct a post-marketing study, monitoring patients receiving the new system for adverse events and metal ion concentrations in their blood” – post marketing – how about before they sell it widely so that the world in not subjected to more suffering human guinea pigs…
- The study was started before DePuy had to recall is ASR hip replacement system – so it is hard to see how they could have incorporated any of the the “learnings” from the current debacle …
- The studies reported in the DePuy attachment are long on all the sort of information that proved irrelevant in the current DePuy ASR hip recall…
The DePuy paper is really short on the stuff that is VERY important in the current DePuy ASR recall:
Summary of Metal Ion Testing
A supplemental investigation was conducted at two (2) investigational centers. Chromium, cobalt, and titanium ions were measured preoperatively, and at 3 months, 12 months and 24 months postoperative. Blood samples were taken at these intervals, and separated into serum and erythrocytes. Each of these sample types was tested for chromium, cobalt, and titanium ion levels. In addition, urine was tested for chromium and cobalt ion levels, but not for titanium. Results were reported in parts per billion (ppb), equivalent to μg/l. Median ion results were low at every time interval for each metal tested for investigational and control hips. For urine cobalt ions, median levels were < 3.0 μg/L at each time interval. All of the remaining median ion levels were < 2.2 μg/L at each time interval. There was no difference between treatment groups observed.
Their conclusions were very interesting to say the least …
In conclusion, the results demonstrate the safety and efficacy of the COM total hip system. Efficacy was demonstrated by proving that the investigational devices were not inferior to the control devices using the composite proportion success outcome. Safety was demonstrated by the fact that there was no difference in the proportions of adverse events or in survivorship for investigational compared to control devices.
Perhaps I just need a new course in logic and how to read scientific literature…
And here is where it gets more interesting:
- the metal ion concentrations are reported as median, NOT the average – note: the median is the middle score, when they’re arranged in order. 1,3,(4),4,5. So median = 4 in this case; the average (“mean”) is the sum of all of the numbers divided by the sample size.
- in this case it is possible to conclude, in the absence of the actual data, that they have sought to conveniently let the reader think that this is the “average” which may in fact be considerably higher than the median…
- the press release that sparked this blog said … “the manufacturer will conduct a post-marketing study, monitoring patients receiving the new system for adverse events and metal ion concentrations in their blood” …. so they are going to measure real concentrations after they pocket millions of dollars for trying it on arthritic long-suffering patients…
Cobalt – Clinical comment – source of information – click here:
Blood : Normal blood values are less than 1 μg/L
Serum/Plasma: Normal serum values are less than 0.5 μg/L
Urine: Normal urine values are less than 2 μg/L
Chromium – Clinical comments:
Blood: Normal blood values are less than 0.5 μg/L
Urine: Normal urinary chromium values are less than 1 μg/L
Plasma/Serum: Normal concentrations are usually less than 0.35 μg/L
RBCs: Normal concentrations for soluble Chromium VI compounds is less than 7 μg/L
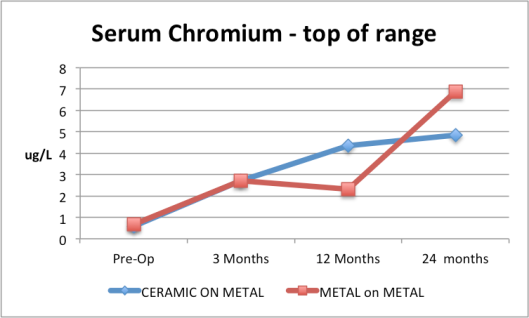
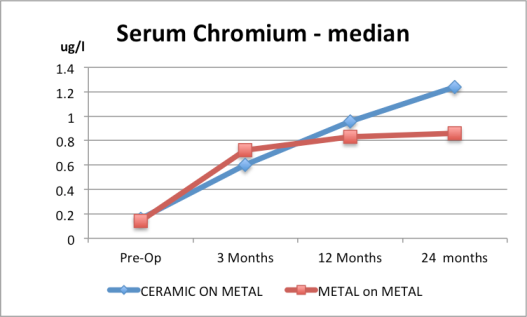
I have found the data submitted to the FDA – here it is in table format followed by some interesting graphs I have prepared from their data.
Pinnacle® CoMplete® Acetabular Hip System – P090002
Issued June 13, 2011
- Approval Order
- Summary
- Labeling
- Other Consumer Information
It is clear that the data are skewed and the failure to report the average result is somewhat damning in my view…

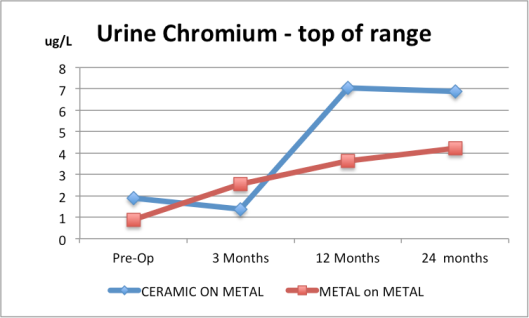
1. Urine Cobalt for the Ceramic and the Metal-on-Metal are both way above normal range based on the top of the ranges given. Normal is up to 2 microgram/L in urine. |
2. Urine Cobalt for the Ceramic and the Metal-on-Metal are both above normal range based on the median of the ranges given. Normal is up to 2 microgram/L in urine. |
3. Urine chromium for the Ceramic and the Metal-on-Metal are both way above normal range based on the top of the ranges given. Normal urinary chromium values are less than 1 microgram/L.
|
4. Urine chromium for the Ceramic and the Metal-on-Metal are both above normal range based on the median of the ranges given. Normal urinary chromium values are less than 1 microgram/L.
|
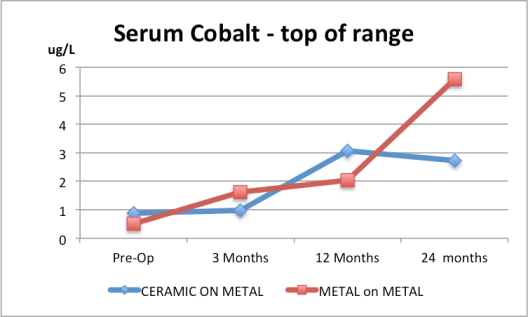
5. Serum cobalt for the Ceramic and the Metal-on-Metal are both way above normal range based on the top of the ranges given. Normal serum values are less than 0.5 microgram/L.
|
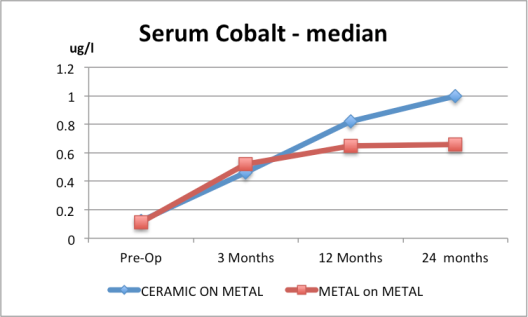
6. Serum cobalt for the Ceramic and the Metal-on-Metal are both above normal range based on the median of the ranges given. Normal serum values are less than 0.5 microgram/L.
|
7. Serum chromium for the Ceramic and the Metal-on-Metal are both way above normal range based on the top of the ranges given. Normal concentrations are usually less than 0.35 microgram/L/L.
|
8. Serum chromium for the Ceramic and the Metal-on-Metal are both way above normal range based on the top of the ranges given. Normal concentrations are usually less than 0.35 microgram/L.
|
And here is something really scary…
the way they attach the ceramic femoral head – with impact!!! How can we be sure that the ceramic head (ceramics are brittle) is not damaged in the process so that micro surface irregularities or micro fractures are not caused which then leads to sharp grinding against the cobalt chrome molybdenum acetabular cup liner – which would release even more metal ions into the hip region…???
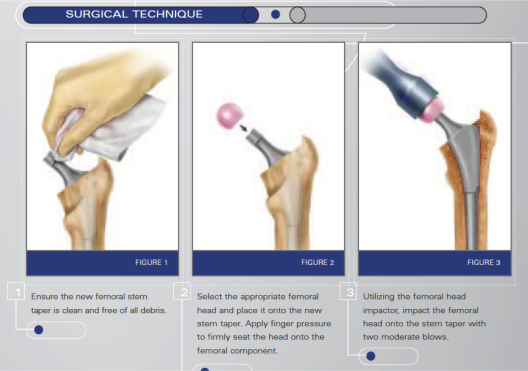
DePuy illustration on how to attach the ceramic femoral head - from DePuy Attachment
This I really don’t understand…
The CoMplete™ System and all implants indicated to be used with the CoMplete™System are proven to be safe and biocompatible with the human body. The CoMplete™ System was studied in a group of 390 patients between August 2005 and November 2008 at eleven hospitals across the United States and Canada. The study was approved by the U.S. Food and Drug Administration. For those patients who finished the study, results showed that 94.2% of the patients with the CoMplete™ System had either good or excellent results from their surgeries as compared to 94.3% of patients who received a conventional hip replacement prosthesis.
I have underlined
…are proven to be safe and biocompatible with the human body.
Am I missing something, the “intuitive leap” to “biocompatible” – do they mean, not too toxic to kill off the host? Biocompatible, I just don’t get – where is the evidence? Or is it the medical marketers at DePuy having a intuitive spasm?
And then there are these BOLD statements!
What Are Potential Benefits of the CoMplete™ Acetabular Hip System vs. Other Systems?
A primary goal of hip replacement parts is to last as long as possible while providing a pain free, more natural range of motion. While there is no guarantee of success, benefits can include relief of pain and the return to a more normal use of the hip. The best way to compare different hip systems is to look at the wear and tear over use. The easiest way to do this is to test different material components on a special machine in a laboratory to see how long the implants last while releasing as little wear debris in a special fluid. Wear debris is created by two components rubbing together over an extended period of time.
The three most common material systems used are Ceramic-on-Ceramic, Metal-on- Plastic and Metal-on-Metal. All three systems have been tested on this special machine to measure the amount of wear debris. It is believed that a system with low wear debris will last longer then one with high wear debris. The CoMplete hip System is a new, fourth option that produces some of the lowest wear debris compared to these other systems. Not only does the CoMplete™ System have very low wear, there is evidence that indicates any ceramic wear particles that are produced are more biocompatible than are metal or polyethylene wear particles and are better tolerated by the human body.
While Metal-on-Metal produces low wear debris, some surgeons will not use this combination for female patients that are at childbearing age or may have a kidney disorder. The CoMplete™ System does not have these limitations.
Their last paragraph has me speechless- their own data shows urine cobalt levels above normal – and they are willing to say that is it OK women who may get pregnant – that is extremely risky I would hazard to suggest… I wonder if their corporate legal counsel has seen this brilliant bit of marketing…
And then they have this to say as well:
Risks:
The risks associated with this hip replacement are expected to be similar to those of other hip replacements. Each of these reactions or complications can arise during an after surgery and may require medical intervention (such as surgery) and implant removal. Once implanted, the functional life of any total hip prosthesis is not clearly known at this time. To reduce the risk for failure, please discuss with your doctor what you should do prior to surgery and carefully follow any instructions given to you. The risks and complications include:
- Excessive wear of the components secondary to damage of mating wear surface or debris particles
- The potential long term biological effects of metal wear debris and metal ion production is not known [note: who makes this stuff up...???]
- Tissue reactions, osteolysis and/or implant loosening caused by metallic corrosion, allergic reactions or the accumulation of metal wear debris
- Potential of pain
- Femoral or acetabular perforation, or bone fracture while seating the device
- Damage to blood vessels resulting in hematoma
- Temporary or permanent nerve damage resulting in pain or numbness of the affected limb
- Undesirable shortening or lengthening of the limb
- Traumatic arthrosis of the hip from intraoperative positioning of the extremity
- Cardiovascular disorders including venous thrombosis, pulmonary embolism, or myocardial infarction
- Temporary or permanent neuropathies
- Delayed wound healing
- Infection Migration, loosening, subluxation, or dislocation of the prosthesis
- Periarticular calcification or ossification, with or without impediment to joint mobility
- Inadequate range of motion due to improper selection or positioning of components, by femoral impingement, and periarticular calcification
- The risk of death
Technorati Tags: arthritis, Articulating Joints, Baby Boomers, ceramic, Elderly Patients, hip implant, hip prosthesis, Hip recall, Hip Replacement, Hip Revision, joint disease, joint replacement, MoM hips, orthopaedic implants, polyethylene wear debris, polymeric, ceramic-on-metal hip, FDA, FDA Appproval, Clinical Trials, second surgery, metal ion concentrations
Related articles
- Dozens suing over DePuy hip replacement (earlsview.com)
- Veterans are Put through Another War – Hip Recall (earlsview.com)
- Rottenstein Law Group Curious About Reserves Set Aside by Johnson & Johnson for DePuy Victims (earlsview.com)
- Crucial Documents Could Determine DePuy Hip Replacement Lawsuit Results (earlsview.com)
- Lawsuit Against DePuy Filed From Spain (earlsview.com)
- Hip-replacement recall hurts profits at Johnson & Johnson (earlsview.com)
- Rottenstein Law Group Curious About Reserves Set Aside by Johnson & Johnson for DePuy Victims (prweb.com)
- Sadwin Doesn’t buy the BHR Story … (earlsview.com)
- Another Legal Victory for Victims With Recalled DePuy ASR Hip Replacement Implants (earlsview.com)
- Sadwin has a few other Pithy Points for the Orthopaedic “Big Guys”… (earlsview.com)




heck your vary negitive . i have a ceramic on metal hip replacement nearly 4 years not a twinge no probs at all so far . metal ions not elivated . the nice thing is its actually a hard on soft bearing the ceramic is harder than the metal so it gives the feeling of give that metal on wear out fast poly particals evreyware . does.just because they had a prob with their metal on metal hip does not mean all mettal on metal articulations are bad . what do you offer your young patients an arthrodesis of the hip i bet . go back to the stone age . overly conservitive orthopeadic surgeons how would we have ever progressed if we relied on their fear and negitivity.
Great – pleased it is working out. I guess when you have 3 years of hell with metal on metal you get a little jaundiced.
All the best with it & I hope all is fine.
Regards
Earl
thanks for your reply i would not take a metal on metal but 28 or 32 metal head on a metal socket seems to work fine its when they make them alot bigger the problems start . its not the metalon metak it was the love of money caused all the problems. rushing products out rather than a full quality control precess being followed . so give up on new tech and take a new look at ceramic on metal , it has the potential to become the replacement of coc or olf fashiond stell on plastic.i guess you have to be more carefull in the states as they take people to court for jack there .even ceramic on ceramic the amount that squeak is just a fraction us younger patients need these implants you would not do a metall on plastic in a 30 year old patient would you .even if it was crosslinked that makess it brittle like ceramic used to be .
Dr. Steve said-
I can state, as an arthroprosthetic surgeon with nearly thirty year of clinical experience, that 98% of patients are as pleased as Bernard is with their hips at four years. Even if (and especially if) they were implanted with a proven, old school, metal-on-plastic hip with a femoral head equal to or less than 32 mm diameter. Replaced hips tend to either “perform” well or not whether they are metal-plastic, metal-metal, ceramic-ceramic, ceramic-metal, or ceramic-plastic.
The best evidence to date (combined joint registry data from multiple countries) indicates that the safest choice for about 99% of patients requiring a new ball joint is a metal head (chrome-cobalt does well as long as you do not rub it on itself or something harder, aka ceramic) and XPLE polyethylene plastic. The XPLE plastic came along about a decade ago and it appears to have solved the wear issues that we had with the older plastic.
The chief of the Aussie total joint registry has done a brilliant study that shows that the standard hip technology of a decade ago is out performing the crap that came after. The arthroprosthetic industry is driven to innovate because newer stuff markets better and they can charge more for it. We have fifteen year proven designs and materials that are not failing due to wear, are relatively simple, and relatively forgiving for human surgeons to install. There is not much logic for leaving less expensive proven success for more expensive unproven marketed latest and greatest that might have a myriad of unknown downside risk.
This truth is an unfortunate one for the arthroprosthetic companies and for design surgeons that are paid millions to dream up something new. A proven metal-on-XPLE hip can be had for a fraction of the cost of a metal-metal (god forbid) or metal-ceramic (likely even worse), or ceramic-on-ceramic one (quite expensive, survives as well as but not better than metal-on-XPLE, ceramic can fracture which is a difficult fix, and the CoCs are noisy in a significant number of patients). Ceramic-on-plastic is a good choice for patients at high risk for a chrome-cobalt metal hypersensitivity or if one is attempting to salvage a failed CoC, MoM, or MoC, this is the common instance in which I use the CoP option.
That Bernard credits his metal-on-ceramic hip for his good result is due to a human logical wiring short circuit called “attributional basis.” Bernard needs to have his cobalt levels checked yearly. He is likely to do well but the worst cases of arthroprosthetic cobaltism reported, including the only fatal one have involved a mix of chrome-cobalt parts and ceramic ones. Ceramic bits are much harder than chrome-cobalt, if Bernard’s very expensive ceramic head chips the resulting abrasive grit will eat his very expensive chrome-cobalt socket alive. When than occurs cobalt levels sore to > 300 mcg/L and patients tend to become deaf, blind, numb, Cretean, and dumb quite quickly (over a period of months!). Fortunately, this is a rare occurrence.
I know attributional basis well as a patient, a surgeon, and a researcher. Up until a year after my ASR I was singing its praises, implanting large head MoMs in my patients, and embracing any wisp of gossip or science that supported my choice of implant.
Surgeons and patients are equally marketable and fallible.
Bernard is statistically likely to have a blood cobalt of 1 to 2 mcg/L, should he develop renal dysfunction as an elder (most do) that level will climb. At that point his GP will have to attempt to determine whether his mood disorder, hypothyroidism, mild heart failure, abdominal lymphoma, and declining memory and Suduko performance are a coincidence or related to years of unremitting and increasing blood cobalt and chromium level well beyond the level of comfort set for an industrial worker and the end of his work week.
In short Bernard, although he is likely to do fine with his MoC but he and society would have likely been better served had he and his surgeon selected a MoXPLE on with a 32 mm ball. The up front bill would have been less for the parts, he would not be troubled by what effects his cobalt and chromium level in the 1-2 mcg/liter range might have other his lifetime, and he or society would not bear the extra 200-5000 dollar per annum cost of the additional metal level testing and or a metal suppression MRI (MSMRI) should he experience any symptoms at the hip.
Bernard. If you still have you’re ceramic on metal Depuy implant in you, send the results to Depuy. They will need some people to try and defend the fact that the whole design is flawed, and you may be one of the few who can attest to it! You should check out my outcome of the “improved” (with no data on humans) implant they slid by the FDA ($$$) on June 14th, 2011. Today my story is published on this site. 2/10/2013–might have a look. All that glitters is not gold. Hope you are in good shape, but lets be honest, there never was any evidence the new Ceramic on Metal would be any better, just “not worse”–that’s not too encouraging to me. Turned out a living hell. I am fairly sure that metal is metal, regardless whether its on the top or bottom, for one thing, and I am 100% sure that ceramic will scrape chunks of metal off without trouble, as a matter of fact I am looking at my scraped up metal and scraped up implant as I type. It was given back to me after 2 surgeries to fix my hips.
I am 3 years, 3 months into a THR with a ceramic on metal Depuy. I have had numbness in my right leg and terrible muscle spasms/pain in the groin hip area. I am 51 years old and feel like a 75 year old who never recovered from hip replacement. I teach arthritis classes in the pool at a local gym, so I have been active and trying to stay positive. However, after reading this article I feel more like a guinnea pig. I have bersitis near the scar and after some physical therapy this week, I couldn’t even put any weight on my leg with the hip replacement. I was limping. I thought the ceramic was the way to go…but now, I’m not so sure.
Hi Karen
That is terrible. What are your metal ion levels? Have you had them measured? What does your surgeon say?
Earl
Hi Earl,
I don’t know my metal ion levels, not sure if that test has been ordered. How often should that be done? My last visit with my surgeon was at 2 yrs and at that time he recommended pain management and said that it (the hip) was probably as good as it’s going to get.
Ok. So here is a problem. You need to have an x-ray and blood tests at least annually. And the pain management thing is just a fob off. With the DePuy ASR and Pinnacle disasters you need to see the possibility that J&J have developed and marketed more than one toxic hip.
There is a recent post on the blog about Timothy Petri who had massive problems with the same hip. I have asked him to email you.
Earl
Also I have asked Dr Stephen Tower, the surgeon who blew the whistle on the ASR to look at your case.
Wow. Okay…thanks so much! My first thought was, why didn’t he give me a ceramic on ceramic? Isn’t it designed for people under 60 who are fairly active? Since the hip replacement I’ve also been disagnosed with hypothyroidism. Look forward to hearing from Timothy Petri. Again thanks for the information.
Karen
Hi Karen
You have to wonder but maybe the surgeon followed the guidance of J&J and had no idea of material science – but try rubbing your kitchen knife on a ceramic plate for a while and see what happens – wear on the ceramic and the knife gets sharper because the metal is rubbed off…
There have been a lot of developments since your surgeon did the operation and hopefully he is an honest one and has learned something from the general DePuy debacle – if not, you may need to find a smart surgeon who is not in the pocket of some big orthopaedic company.
Cheers
Earl
Comments from Dr Steve,
Dear Ms. Eichenbaum,
I am sorry for your troubles.
Ceramic-on-metal hips have the same or greater risk of generating chrome-cobalt metal wear debris around the hip as metal-on-metal hips do.
This can result in tissue destruction about the hip and can result in elevated levels of cobalt and chromium in the blood.
See your surgeon post haste and request a blood cobalt and chromium level and a metal suppression MRI (MSMRI) of your sore hip.
Even if your metal levels are not elevated you might be having a reaction to a small amount of chrome cobalt debris.
Some patients are just very sensitive the chrome cobalt wear or corrosion products. I have had several patients with severe damage to the tissues about the hip that did not have elevated blood metal levels.
Numbness of the same leg might indicate a large pseudotumor that is pressing on the nerves or high levels of metal in the tissues about the nerves, cobalt in particular has the potential to cause nerve damage.Both
If your surgeon trivializes your problems find another or ask your primary medical provider to order the metal levels and the MSMRI.
If your blood cobalt level is > 7 mcg/L and is still high when repeated several months down the pike your hip likely ought to be revised to a ceramic-on-plastic bearing, if the stem is made of chrome cobalt it ought to be switched to a titanium one as well.
If your blood cobalt level is > 7 mcg/L your primary provider should also evaluate your mood, cognition, hearing, sight, thyroid (including antibodies), and your heart.
Let us know how things turn out or if I can be of further help.
Sincerely,
Dr. Steve
Hi Dr. Steve,
The numbness behind my knee was attributed to sciatica by hospital and doctor. But it didn’t occur until AFTER the hip replacement. I am shocked by all this, I thought I was maybe going through “the change” of life, even though I still menstrate because of the crying uncontrollably and not really having a reason. I’ve also been breaking out in randon rashes on my arms after teaching (exercise) in the pool. Thought I was developing an allergy to the pool water. I’ve been getting headaches almost daily and just not feeling well. Hip and groin pain has gotten worse over time, along with my legs and muscles twtching.
My question is…why are doctors implanting these devices and in my case…before it was even approved?
Nevermind…it doesn’t matter and I think Earl answered that question anyway. I can’t believe J&J based their studies on a hip that was getting ready to be recalled. Just doesn’t make sense to me. I am so mad, however, I will be taking your advice and appreciate all your help and the information.
Thanks again,
Karen
Here’s the update:
Saw my surgeon’s assistant today and he took x-rays and said they looked ok. I asked him for the metal ions blood test and he said they needed to test for an infection, and that he didn’t think this hip had a problem with metal ions..it was the metal on metal hip that had that problem. When I asked him if he knew J&J based their studies for THIS hip on the MOM hips, he said, I don’t think they knew at that time that the MOM hips were going to be recalled…Seriously?! I find that hard to believe! Anyway, he said IF my white cell count is up then they would have to aspirate the hip which sounds very painful. We should know by Monday about the bloodwork, and then he said I may need a bone scan, not a MRI.
Hi Karen – stick to your guns – as Dr Steve said ceramic on metal will generate metal ions. There are a lot of qualified people out there who think they know everything – but they know little. Bone scan is OK but MRI’s are very good.
Earl
Hi Earl,
They called late today and said blood work came back with no infection in my hip or anywhere in my body. Thank goodness for that. So they will set up the bone scan and call me. I am going to insist on the metal ions test..and if they won’t do it, I’ll get my primary care doctor to. I’m certain all this pain in and around my hip cannot be normal. He informed me that I could not have a MRI because I have metal in my hip but they will run a bone scan.
He also suggested I talk to my back surgeon because my back pain has increased since a fusion L5-S1 in 04. However, it seems that the pain has increased more in the last 3 years.
OK – well that is good to rule out too. I am struggling to figure out what level of pain is normal and acceptable vs. not from a personal standpoint. My revision x-rays look fantastic but the hip still has some unhappy days – but as my surgeon said to me, two operations in 3 years of a major nature disrupts the hip capsule, muscle etc. All part of life’s rich tapestry!
Earl
I’m sorry you have been through so much. Although, look at the positive, you have enlightened, and in some cases, probably saved people’s lives because of your experience. I know that’s a small consolation for all the pain and suffering you’ve been through, but you have to realize the enormity of what you are doing and the good. ) I’m still processing all this and struggling with how crappy I feel every day. I called my doctor’s office again on Friday to inquire about the metal ions test…no return call. I guess at this point, they are going to call my insurance and inquire on the bone scan. Maybe I’ll call tomorrow and inquire myself and ask my insurance what I need to do to get this removed? I’m not staying in all this pain and feeling my teeth ache, headaches, body aches, anxiety, etc., it’s unacceptable.
Update: Went to primary care doctor yesterday and asked him to do the metal ions blood test. He agreed. Should know the results by Friday. Surgeons office returned my call from Friday. Told him I wanted the metal ions test, but since they didn’t return my call, I went to my Primary care doctor and had him do it. He then said they would set up the bone scan, which is planned for Monday and Indium White Blood scan on Tues.