Tags
ASR XL, Biomet, chromium levels in the bloodstream, cobalt-chromium, defective hip replacement devices, DePuy, DePuy ASR™ Hip Resurfacing System, DePuy ASR™ XL Acetabular System, DePuy Orthopaedics, DePuy Pinnacle System, Durom, FDA review, femoral head, Food & Drug Administration, Hip recall, Hip Replacement, hip replacement surgery, hip resurfacing, joint replacement, Metal-on-Metal components, Metal-on-Metal DePuy hip device, Metasul LDH, Microscopic metal particles, non-functioning hip implant, Orthopedic surgery, Patient, revision surgery, second hip replacement surgery, Smith and Nephew, surgery, U.S. Food and Drug Administration, Ultamet, Viles & Beckman, Wright Medical, Zimmer, Zimmer Durom
Total Hip Arthroplasty: The Defects, the FDA and how Patients are Harmed
©Viles & Beckman, LLC, 6350 Presidential Court Fort Myers, FL 34135, (239) 334-3933 http://www.vilesandbeckman.com
My View
This has to be one of the best written summaries of the overall problems with Hip Replacements. It is a Must Read. Download it as a PDF here – click here
Introduction
Katie Korgaokar of Denver is a living example of one of the most common, effective and sometimes, problematic surgical inventions of the 20th century – the hip replacement. A congenital condition, Perthes disease, had prematurely deteriorated the bones in her hip joint, compelling Korgaokar to seek a total hip replacement.1 “The initial hip replacement surgery was a huge success,” she testified to the Senate Committee on Aging. “Within three months of the surgery, I was essentially pain free and was able to engage in activities that had previously been off limits. The surgery truly changed my life.”2 Her surgeon told her that her new, Metal-on-Metal DePuy hip device would last 20 years.
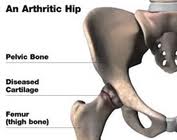 Instead, Korgaokar found herself back on the operating table just four years later. Her state-of-the-art hip had been recalled, and state-of-the-art hip showed that metal wear particles from the hip had elevated the level of cobalt and chromium in her bloods stream by 1,000 percent above normal levels. Her revision surgery was more troublesome, and in April 2011, when she testified during a hearing on the U.S. Food and Drug Administration regulation and oversight of medical devices, she was still in pain and only recovering her mobility slowly.3 Orthopedic surgeons have been performing hip replacement and hip resurfacing operations for nearly a century.
Instead, Korgaokar found herself back on the operating table just four years later. Her state-of-the-art hip had been recalled, and state-of-the-art hip showed that metal wear particles from the hip had elevated the level of cobalt and chromium in her bloods stream by 1,000 percent above normal levels. Her revision surgery was more troublesome, and in April 2011, when she testified during a hearing on the U.S. Food and Drug Administration regulation and oversight of medical devices, she was still in pain and only recovering her mobility slowly.3 Orthopedic surgeons have been performing hip replacement and hip resurfacing operations for nearly a century.
Each year, about 300,000 patients in the U.S. undergo hip arthroplasty, and many consider this procedure to be “the major success story in orthopedic surgery in the twentieth century.”4 But hip implants have their failures. All hip implants are plagued by the problem of wearing away of the components caused by friction. Even normal wear can cause the implant to loosen and release microscopic particles into the patient’s body that have a multitude of short-term and long-range adverse effects. The patient experiences these problems as pain, diminished movement and another trip to the orthopedic surgeon for a second hip replacement surgery. Excessive wear, created by designs that do not tolerate even a slight misalignment when implanted speeds up and multiplies the damage to the patient’s health.
 A recent return to Metal-on-Metal (MoM) hip replacement devices, after decades of experimentation with other materials, has brought about a fresh wave of adverse effects necessitating revision surgeries. Microscopic metal particles released from the metal components as a result of friction and wear can raise cobalt and chromium levels in the bloodstream, damage and destroy surrounding tissue and can be genotoxic to patients. In 2010, surgeons performed 50,000 revisions – meaning a surgery to replace an old, defective or non-functioning hip implant – accounting for 16.5 percent of hip replacements each year.5 In the case of defective implants, many of the defects aren’t discovered until well after the device has been in use and implanted in hundreds of patients. Although medical devices have been regulated by the U.S. Food and Drug Administration since 1976, manufacturers have taken advantage of rules streamlining the approval process to bring bad designs and material combinations into the marketplace with no clinical testing or proof of efficacy.
A recent return to Metal-on-Metal (MoM) hip replacement devices, after decades of experimentation with other materials, has brought about a fresh wave of adverse effects necessitating revision surgeries. Microscopic metal particles released from the metal components as a result of friction and wear can raise cobalt and chromium levels in the bloodstream, damage and destroy surrounding tissue and can be genotoxic to patients. In 2010, surgeons performed 50,000 revisions – meaning a surgery to replace an old, defective or non-functioning hip implant – accounting for 16.5 percent of hip replacements each year.5 In the case of defective implants, many of the defects aren’t discovered until well after the device has been in use and implanted in hundreds of patients. Although medical devices have been regulated by the U.S. Food and Drug Administration since 1976, manufacturers have taken advantage of rules streamlining the approval process to bring bad designs and material combinations into the marketplace with no clinical testing or proof of efficacy.
 Since 2008, three defective hip replacement devices have been recalled, after implantation in some 100,000 patients in the U.S.: Since 2008, two manufacturers, DePuy Orthopaedics and Zimmer, have suspended sales of three different MoM devices, after doctors reported high failure rates. The DePuy ASR™ XL Acetabular System and DePuy ASR™ Hip Resurfacing System and the Zimmer Durom® had been implanted in some 100,000 patients before sales were suspended. And the complaints to the FDA continue. In 2011, there were thousands complaints. Since 2005, the patients and surgeons have lodged more than 800 complaints involving an unrecalled Metal-on-Metal device, the DePuy Pinnacle System. From 2008 to 2010, those complaints quadrupled. These reports are not surprising given that DePuy sought FDA approval for the recalled ASR system by claiming that its design was substantially similar to the Pinnacle Metal-on-Metal components.
Since 2008, three defective hip replacement devices have been recalled, after implantation in some 100,000 patients in the U.S.: Since 2008, two manufacturers, DePuy Orthopaedics and Zimmer, have suspended sales of three different MoM devices, after doctors reported high failure rates. The DePuy ASR™ XL Acetabular System and DePuy ASR™ Hip Resurfacing System and the Zimmer Durom® had been implanted in some 100,000 patients before sales were suspended. And the complaints to the FDA continue. In 2011, there were thousands complaints. Since 2005, the patients and surgeons have lodged more than 800 complaints involving an unrecalled Metal-on-Metal device, the DePuy Pinnacle System. From 2008 to 2010, those complaints quadrupled. These reports are not surprising given that DePuy sought FDA approval for the recalled ASR system by claiming that its design was substantially similar to the Pinnacle Metal-on-Metal components.
Last year, the FDA began to address the longstanding complaints of consumers, the medical community and Government Accounting Office investigators that the approval process for implantable devices was too lax. But, the FDA’s failure to act on the Pinnacle Metal-on-Metal system means that the agency still has a way to go. Meanwhile, recent studies show that the rate of recalls for medical devices that could cause serious injury and death is much higher for those that were made available for sale through an expedited FDA review.
Total Hip Arthroplasty Overview
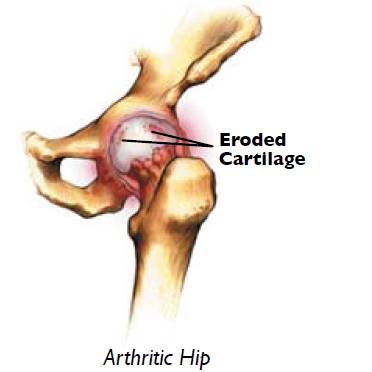 Today two reconstructive procedures performed everyday, worldwide: Total Hip Arthroplasty (THA) and Hip Resurfacing Arthroplasty (RSA). Developed in the 1920′s, RSA involves replacing only the damaged bearing surfaces of the hip joint, rather than the entire femoral head.6 Total Hip Arthroplasty, a newer iteration of hip replacement surgery, has been performed since 1956.7 Current Total Hip Replacements consist of three basic components:
Today two reconstructive procedures performed everyday, worldwide: Total Hip Arthroplasty (THA) and Hip Resurfacing Arthroplasty (RSA). Developed in the 1920′s, RSA involves replacing only the damaged bearing surfaces of the hip joint, rather than the entire femoral head.6 Total Hip Arthroplasty, a newer iteration of hip replacement surgery, has been performed since 1956.7 Current Total Hip Replacements consist of three basic components:
- The acetabular cup is typically made of metal and can have a liner made of polyethylene, ceramic or metal
- The ball, which replaces the head of the femur, is typically made of cobalt-chromium (CoCr) alloys, stainless steel or ceramic materials (aluminium oxide or zirconium oxide)
- The stem, which fits into the femur, is typically made of titanium alloy (Ti6Al4V), CoCr, or stainless steel.
Orthopaedic device manufacturers have been working with hip implants for almost a hundred years. Some have been robust lasting for more than 10 years in patients, but others have had much shorter life spans than advertised and present serious adverse affects to patients. The key to a THA’s long-term success is the robustness of the bearing surface: the femoral head and its interaction with the acetabular lining and the anatomical functionality after implantation. In general, there are four device options for the bearing surface in total hip replacement:
Hard-on-Soft Combinations:
- ” Metal-on-Polyethylene (MoPE): ball is made of metal and the acetabular liner is polyethylene
- ” Ceramic-on-Polyethylene (CoPE): ball is made of ceramic and the acetabular liner is polyethylene
Hard-on-Hard Combinations:
- ” Metal-on-Metal (MoM): The ball and acetabular liner are both made of metal.
- ” Ceramic-on-Ceramic (CoCr): The ball and the acetabular liner are both made of ceramic.
Metal-on-Metal Hip Replacements
 In modern Metal-on-Metal hip implants, the bearing surfaces – the femoral head and acetabular cup liner – are made of cobalt and chromium, which offer high wear resistance and less debris as measured by volume than metal-on-polyethylene implants. These alloys are extremely hard, resistant to corrosion and can be machined to a smooth surface. They are self-polishing, so small defects tend to smooth out over time. The very first iterations of total hip replacements were Metal-on-Metal designs.
In modern Metal-on-Metal hip implants, the bearing surfaces – the femoral head and acetabular cup liner – are made of cobalt and chromium, which offer high wear resistance and less debris as measured by volume than metal-on-polyethylene implants. These alloys are extremely hard, resistant to corrosion and can be machined to a smooth surface. They are self-polishing, so small defects tend to smooth out over time. The very first iterations of total hip replacements were Metal-on-Metal designs.
Contemporary Metal-on-Metal hip replacements in the marketplace today include:
- ASR XL (DePuy)
- Pinnacle Hip System with the Ultamet Liner (DePuy)
- Metasul LDH (Zimmer)
- M2aMagnum (Biomet)
- Conserve (Wright Medical)
- Lubrimet (Smith and Nephew)
- Durom (Zimmer)
Manufacturers and surgeons have promoted Metal-on-Metal hip implant systems for younger and physically active patients because the metal is more durable than polyethylene and produces less wear debris, as measured by volume. The component which confers the benefits of a Metal-on-Metal implant is the large femoral head, which can be used because less room is taken up by the acetabular liner. The large-diameter femoral heads reduce the risk of dislocation, increase the range of motion, decrease the risk of impingement and reduce wear production. Surgeons and manufacturers have postulated that this type of bearing is good for patients who will demand more function from the implant, patients at risk for dislocation and younger patients who require an implant that will last longer. Manufacturers, or researchers sponsored by hip implant manufacturers, have published literature espousing those potential benefits.
For example, DePuy Orthopaedics, a Warsaw, Indiana manufacturer makes these claims in a number of brochures. In High Stability, Low Wear Metal-on-Metal Bearings: Benefits, Risks and Alternatives, the manufacturer cites: “large diameter bearings have greater stability” and, “when properly positioned the wear rate has been documented to be very low in vivo for three decades.”8 A DePuy Benefits of Metal on Metal brochure advertises: “durability,” “strength and fracture resistance; resistance to wear;” “increased range of motion and maximized jump distance and stability.”9 Mechanical simulation studies have supported manufacturers’ contention that MoM implants are effective.10 11 These types of studies are conducted under tightly-controlled laboratory conditions with the implant in the precisely proper orientation. A hip joint simulator is a machine that moves the femoral component forward and back, or side to side to side – movement that simulates walking, running, jumping and other human hip functions.
A series of simulator studies in 1999 generally showed the Metal-on-Metal implants produced less wear, as measured by volume, than metal-on-polyethylene designs. One compared the friction, lubrication and wear performance of Metal-on-Metal systems to metal-on polyethylene systems.12 This study, and others, found that the MoM bearings generated 100 times less wear debris, by volume, than the metal-on-polyethylene systems. However, metal particles are much smaller than polyethylene particles. The number of metal particles was 1,000 times greater than those generated by a polyethylene bearing even though the total wear, as measured by volume, was lower.13 14 Some randomized clinical trials comparing conventional metal-on-polyethylene total hip replacements to Metal-on-Metal implants have also demonstrated favorable short to medium-term results for the MoM.15 16 17 18 Long-term retrospective observations of patients with Metal-on-Metal implants have also demonstrated long-term survivorship and good mechanical performance.
The U.S. Food and Drug Administration estimates that the Metal-on-Metal device survival-range for non-defective, properly implanted devices at 95.5 percent at twelve years to 93 percent at ten years.19 However, their effectiveness has been highly over-rated in the literature and by FDA. Even with superior laboratory wear rates, retrieval studies documenting midterm revision rates do not show that second generation MoM systems last longer than metal-on-polyethylene hip implants. From 2008 to 2010, the Australian National Joint Registry, which reports the effect of the bearing surface on the early to mid-term outcomes for primary conventional total hip replacement, has found that the bearing surface with the highest revision rate is Metal-on-Metal.20 Revision rates for Metal-on-Metal systems are statistically worse than metal-on-polyethylene systems for all sized heads.
The Australian joint registry data show that polyethylene bearing surfaces had the lowest risk of revision compared to all bearing surface types.21 In March 2011, the British Orthopaedic Association announced at the British Hip Society Annual Conference that large diameter MoM bearing devices show a higher than anticipated early failure rate ranging from 21 percent revision rate at 4 years to 12-15 percent at 5 years. The association reported that the DePuy ASR XL had 49 percent revision rate at 6 years.
Toxicity and Metal-on-Metal Bearings
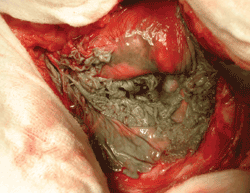 Besides the wear problem, Metal-on-Metal bearings pose a significant health risk to patients even when they are properly implanted and non-defective. In contemporary Metal-on-Metal hip joint bearings, tiny metal debris particles can be shed from the femoral head and acetabular cup surfaces and spread through the body and the bloodstream in the form of metal cobalt and chromium ions. While the metallic ions can be dissolved in the body fluid and eliminated from the patient’s body, (polyethylene wear particles cannot) some patients have developed a range of adverse reactions.22 Metallosis, also known as Adverse Local Tissue Reaction, has been defined by Dr. Schmalzried, who helped develop the DePuy Pinnacle Systems, as “Biologic reactivity to metal particles and the presently unknown long-term effects of exposure to cobalt and chromium ions released from MoM articulating surfaces.”23 24 They are catch-all terms to describe adverse reactions, which can range from effects in the immediate vicinity of the implant to those that affect the entire body. Metal reactivity, from high wear, causes a foreign-body (macrophage) inflammatory response to excessive metal particles. Metal reactivity can impact the entire body. Metal sensitivity is an allergic response that can occur from normal or high wear. Typically, patients experience extreme pain due to the production of cloudy fluid, expanded and ulcerated joint capsule usually encased in fibrous tissue.
Besides the wear problem, Metal-on-Metal bearings pose a significant health risk to patients even when they are properly implanted and non-defective. In contemporary Metal-on-Metal hip joint bearings, tiny metal debris particles can be shed from the femoral head and acetabular cup surfaces and spread through the body and the bloodstream in the form of metal cobalt and chromium ions. While the metallic ions can be dissolved in the body fluid and eliminated from the patient’s body, (polyethylene wear particles cannot) some patients have developed a range of adverse reactions.22 Metallosis, also known as Adverse Local Tissue Reaction, has been defined by Dr. Schmalzried, who helped develop the DePuy Pinnacle Systems, as “Biologic reactivity to metal particles and the presently unknown long-term effects of exposure to cobalt and chromium ions released from MoM articulating surfaces.”23 24 They are catch-all terms to describe adverse reactions, which can range from effects in the immediate vicinity of the implant to those that affect the entire body. Metal reactivity, from high wear, causes a foreign-body (macrophage) inflammatory response to excessive metal particles. Metal reactivity can impact the entire body. Metal sensitivity is an allergic response that can occur from normal or high wear. Typically, patients experience extreme pain due to the production of cloudy fluid, expanded and ulcerated joint capsule usually encased in fibrous tissue.
Whether caused by reactivity or sensitivity, the metal particles around the implants can cause serious damage to bone and/or tissue surrounding the implant and joint, including:
- Necrotic (dead) tissue mass or pseudo-tumor
- Fluid in the joint
- Osteolysis
- Tissue and bone necrosis (bone death)
- Hypersensitivity to metal
Adverse reactions that affect the entire body include:
- Decreased total lymphocyte, which defends against tumors and virally infected cells
- Decreased CD8+T cells, which fight intracellular pathogens and malignancies
- DNA changes
- Chromosomal aberrations
Long-term toxicity of MoM is unknown, but doctors have expressed concerns about the exposure to high levels of cobalt and chromium ions and there are numerous reports showing significant affects on organs away from the articulation. There are isolated case reports on a small number of patients in which high levels of metal ions in the bloodstream may have caused other types of symptoms or illnesses elsewhere in the body, including effects on the heart, nervous system, and thyroid gland. For example, a study published in 2010 documented two cases in which the patient developed cobaltism in reaction to the metal wear particles generated from a hip arthroplasty, shortly after implantation. The normal cobalt level in the bloodstream is 0.19 micrograms per liter. Any level above 1 microgram per liter indicates excessive cobalt exposure. Levels greater than 5 micrograms per liter are considered toxic.25 In these cases, one patient had 122 microns/liter after 36 months; the cobalt level in the other patient’s blood was 23 microns/liter after one year. In excess, cobalt blocks cellular metabolism and can damage multiple organs.
Cobaltism can cause tinnitus, vertigo, deafness, blindness, optic nerve atrophy, convulsions, headaches, peripheral neuropathy, cardiomyopathy, hypothyroidism.26 27 In these patients, high cobalt levels caused reactions to metal debris beyond the tissues surrounding the hip prosthesis.28 In a 2010 report, Britain’s Medicines and Healthcare products Regulatory Agency’ (MHRA) Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment concluded that there was good evidence for an association between hip implants with metal components and increased genotoxicity in patients.29 Specifically, the committee found evidence of genetic damage in patients with certain metal hip implants.
Design and Surgical Technique
Design and surgical technique are keys to both the short term and long term success of hip joint replacements. As clearly demonstrated above, particulate material produced by implant surface wear is the demon, causing both early failures and long term complications. In hip replacement designs, proper orientation and fixation of the femoral and acetabular components are the most important concepts to minimize wear of the devices. Component orientation becomes even more important with hard-on-hard bearings such as MoM and CoC, much more critical than with MoPE bearings. In certain designs, such as DePuy’s ASR and Pinnacle [Metal-on-Metal] Systems (as well as Zimmer’s Durom Cup and Smith and Nephew’s BHR), acetabular cup orientation slightly out of the optimum range has catastrophic results.
While other designs are much more forgiving to alignment issues commonly seen in surgery, DePuy’s ASR and Pinnacle [Metal-on-Metal] Systems exhibit extreme wear under these circumstances that will result in catastrophic failure and extreme danger to the patient. In these designs, extremely high wear rates result from slight increases in abduction angle, smaller femoral head size and large diametrical clearance. This is exaggerated in designs such as the ASR and Pinnacle [Metal-on-Metal] Systems with a low femoral head coverage angle.30 31 32 33 34 35 36 37 38 39 40 41 42 43 Implant designs vary in the amount of hemispherical coverage of the femoral head provided by the acetabular cup liner usually ranging from 165-180 degrees.44 45 46 In these suboptimal designs, the most important preventative measure to take in order to minimize wear is to know the design characteristics and limitations of the components and orient the components in an optimal position for these limitations.47
Some researchers argue that suboptimal acetabular cup placement is preventable with surgeon training.48 However, if a product repeatedly fails due to slight variations in implantation orientations, the problem is with the design, not the surgeon and re-engineering the design is necessary. Rather than correct the problems with its designs, DePuy for example, attempts to put the onus on surgeons. Its product literature stresses the importance of proper acetabular cup placement for its Pinnacle MoM [Metal-on-Metal] systems. Specifically, the company emphasizes that acetabular cup abduction greater than 55 degrees and/or excessive combined anteversion predisposes to edge loading and dangerously high wear rates.49 50 Despite this warning, clinical and simulation studies of DePuy MoM hip implants and studies funded by DePuy document high wear rates and high patient ion levels associated with acetabular cup inclination angles greater than 50 degrees and anteversion angles greater than 29 degrees in MoM bearing surfaces.51 52 53 54 55 56 57 58 59 61 62 63 64 This suggests that the design is too unforgiving of slight misalignment either from initial placement or expected migration within the joint space – unlike other devices, which allow greater tolerance for the position of the component.
The Data
Complaints
It is very difficult to determine the success or failure rates of a particular hip arthroplasty device. Unlike Britain or Australia, the U.S. government does not sponsor a joint registry, in which surgeons and hospitals submit data on primary surgeries and revisions. In 2007, health care conglomerate Kaiser Permanente launched its own National Total Joint Replacement Registry (TJRR), a “database designed as a post-market surveillance system for elective total hip and knee replacement.”65 The registry collects uniform data at the point of care regarding patient demographics, implant characteristics, surgical techniques, and clinical outcomes, in an effort to identify problems and best practices, contain costs and improve care. More than 350 surgeons and 50 hospitals contribute to the database. (As of March 31, 2007, the TJRR recorded 16,945 primary total hip arthroplasties and 2144 revisions – 12.65 percent.)66 Publicly available information, however, is scant. For example, in August 2010, Iowa Senator Charles E. Grassley asked Zimmer Holdings and DePuy Othopaedics to disclose how they tracked the long-term performance of their devices, after reading a New York Times article which reported on the breakdown in relationships between consulting surgeons and device manufacturers, when doctors report problems that go unheeded by their employers.67
The FDA does operate a web-accessible complaint database. Complaints related to hip prostheses get filed into the Manufacturer and User Facility Device Experience (MAUDE) database. MAUDE data consist of voluntary reports made to the FDA since June 1993, user facility reports since 1991, distributor reports since 1993, and manufacturer reports since August 1996.68 While MAUDE data are not intended to be used to evaluate rates of adverse events involving medical devices or to compare adverse event occurrence between products, they are useful for tracking trends in defects associated with devices. Further, MAUDE represents the only government repository of adverse event reports related to medical data. When a hip device is recalled, the company, or other entities may disclose revision rates. For a product that has been suspected of or alleged to have defects, MAUDE can shed some light on complaint trends. For example, DePuy revealed higher than normal revision rates for two DePuy Metal-on-Metal hip devices that the company recalled last year, the DePuy ASR™ XL Acetabular System and the DePuy ASR™ Hip Resurfacing System.
In 2005, DePuy sought and was granted approval for the DePuy ASR Modular Acetabular Cup System by claiming that the design was substantially similar to the DePuy Pinnacle® Metal-on-Metal Acetabular CupLine.69 In January 2008, the company again sought and gained approval for another variation on the ASR metal acetabular cup, the DePuy ASRTM 300 Acetabular Cup System by claiming that the device was “substantially similar” to the DePuy Pinnacle® Acetabular Cup System.70 Despite the company’s claims to the FDA that the metal acetabular cups in the ASR and Pinnacle hip systems are “substantially similar,” DePuy’s Pinnacle hip replacement has not been recalled. In the meantime, a rising number of complaints about the Pinnacle Metal-on-Metal system continue to be lodged by patients and surgeons in the MAUDE system. MAUDE data from 2005 to March 2011 were examined for reports of adverse events involving Pinnacle Metal-on-Metal systems. Records were identified using device codes, keywords, and brand and generic names; see Appendix A for a detailed explanation of that process. Adverse event reports have steadily increased since 2005; Table 1 provides a breakdown of the number of event reports by year.
Table 1. Adverse Report Events Reported in MAUDE by Year Involving DePuy Pinnacle [Metal-on-Metal] Systems
Calendar Year Unique Reports
| Calendar Year | Unique Reports |
| 2011 | 119 (5/13/2011) |
| 2010 | 329 |
| 2009 | 148 |
| 2008 | 86 |
| 2007 | 38 |
| 2006 | 25 |
| 2005 | 15 |
Complaints have risen sharply since 2005 – nearly quadrupling between 2008 and 2010. The narratives that accompany these adverse reports attest to the classic symptoms of defective Metal-on-Metal implants: “Patient was revised to address excessive cup ante-version resulting in dislocation. Metal debris was found in the joint.”71 “Patient had to have revision hip replacement surgery to remove a Metal-on-Metal total hip implant which had caused significant synovial inflammation and metallosis reaction. The implant was a DePuy pinnacle Metal-on-Metal hip replacement system -not the recalled asr system-. Dates of use: (b)(6) 2003 – (b)(6) 2010. Diagnosis or reason for use: hip arthritis. Event abated after use: yes. This is to report a significant problem with complications due to Metal-on-Metal wear particles leading to a pt necessitating revision hip surgery and removal of implants due to severe synovitis and perivascular lymphocytic infiltrate, confirmed with tissue pathology. The relevance is that this is a different product within the same company -DePuy Orthopaedics- which has recently had a different Metal-on-Metal hip implant recalled by the FDA – the ASR hip system-. This is to report a problem with their Pinnacle Metal-on-Metal system.
The common factor being the Metal-on-Metal bearing, leading to metal particulate release, and significant synovial reaction, and necessitating revision surgery and implant removal. Patients should be made aware of the significant risk associated with this system.”72 They also aptly describe the effect on patients: “I had a left hip replacement (DePuy Pinnacle [Metal-on-Metal]) on (b)(6) 2009 and ever since (b)(6) 2010 I have had a problem which keeps getting worse. I have difficulty standing still for any length of time. If I stand for 10 minutes shaving, I am in severe pain for the rest of the day. I can only sleep on my right side. I cannot sleep on my back or left side at all because the pain is too much. I cannot food shop, clothes shop or shop period because I cannot walk for any length of time. Unless my activity includes sitting such as the movies, out to dinner or the doctor’s office, I cannot go out. The quality of my life has deteriorated. I am in worse shape now then I was before I got my hip replaced.”73
Recalls
To date, two Metal-on-Metal total hip replacements and one Metal-on-Metal hip resurfacing system have been recalled in the U.S.: the DePuy ASR™ XL Acetabular System and DePuy ASR™ Hip Resurfacing System in 2010 and the Zimmer Durom® Acetabular Component in 2008. But the problems regarding Metal-on-Metal hip implants first surfaced in Great Britain several years earlier, when orthopaedic surgeons there alerted the regulatory authorities to the problems of metal wear debris.
DePuy ASR™ XL Acetabular System and DePuy ASR™ Hip Resurfacing System Recalls
The roots of the DePuy recalls go back to September 2005, when the British Orthopaedic Association (BOA) contacted the Medicines and Healthcare products Regulatory Agency (MHRA) to express concerns about the ill-effects of metal-wear debris in MoM hip implants.74 Researchers had been documenting evidence that the MoM implants may be genotoxic to their hosts – meaning that the microscopic shreds of chromium and titanium released into the bloodstream was causing genetic damage. The MHRA presented the genotoxicity issue to the Committee on the Safety of Devices (CSD), which, turned to the Department of Health’s independent expert advisory committee, the Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment (COM) for further review.75 After discussing the possible biological and clinical effects produced by metal wear debris generated from hip implants, the COM concluded in July 2006 that there was “there was good evidence for an association between CoCr-on-CoCr and CoCr or TiAlVon- polyethylene hip replacement devices and increased genotoxicity in patients.”76
The COM was less certain about how that genetic damage might impact the health of patients – particularly in the form of an increased risk of cancer. Based on the research current at the time, the COM asserted that “it was not possible to make any definite conclusions as to which metal ions, or interactions between metal ions or particulate metals might be responsible for the observed genotoxicity.”77 The MHRA seconded those sentiments in a companion statement. “Although there is currently no information to suggest that the identified genotoxicity poses a significant health risk, this finding clearly requires further investigation.”78 The Committee on the Safety of Devices decided to set up an Expert Advisory Group, consisting of orthopaedic surgeons, pathologists, toxicologists, immunologists, material scientists, manufacturers, a lay member of the CSD, and relevant MHRA members of staff to assess the clinical significance of the COM findings and to put these into a risk-benefit context.
The Expert Advisory Group continued to meet, issuing a final report in March 2010. In March 2010, DePuy Orthopaedics issued a Field Safety Notice after receiving new data from the UK that demonstrated the ASR™ System had a higher than expected revision rate at 8-9 percent at three years when used with smaller head sizes (less than 50 mm diameter). On August 24, 2010, the Johnson & Johnson subsidiary, announced a voluntary recall of the DePuy ASR™ total hip system after unpublished data from the National Joint Registry of England and Wales indicated that the revision rate for that total hip replacement system was 13 percent (one in eight patients) within five years of implantation and about 12 percent for its hip resurfacing system.79
“The DePuy ASR™ Hip Resurfacing System was only approved for use outside the U.S. and the ASR™ XL Acetabular System was available worldwide.”80 However, more recently, British surgeons have reported a much higher revision rate than noted in the recalls. On March 9, British Orthopaedic Association, the British Hip Society and the Expert Advisory Group on Metal Bearing Hips issued a statement on research presented at a professional conference showing a much higher early failure rate for the ASR XL device. Four sets of unpublished data showed rates ranging from 21 percent after four years, and “potentially rising to 35 [percent] if all currently known painful implants progress to revision,” to 49 percent after six years.
The warning, issued by John Hodgkinson, president of the British Hip Society, John Skinner Chair of the advisory group and Peter Kay, president British Orthopaedic Association, also noted that “other devices have a revision or impending revision rate of 12 – 15 [percent] at 5 years.”81 British surgeons maintained that the potential failure points included “the trunnion at the ‘Morse’ taper where the large diameter Metal head attaches to the stem,” wear on the bearing surface or corrosion on the stem, if uncemented. The failure modes include “loosening of the acetabular component, loosening of the femoral component or metal reaction with necrosis and soft tissue damage as previously seen in a small number of metal on metal hip resurfacing (HR) devices. Failures seem to be more frequent in females.”82 Symptoms of a failed hip replacement included pain and “radiographic changes as seen on plain films, including loosening and lysis. Blood Cobalt and Chromium ions are often, but not always elevated. Ultrasound and MRI scans may show fluid collections, cystic and/or solid masses.”83
Zimmer Durom® Acetabular Component Recall
On July 22, 2008, Zimmer voluntarily – and briefly – withdrew the Durom® Acetabular Component , known as the Durom Cup, from the marketplace, after one of the company’s consulting surgeons, Dr. Lawrence Dorr, “publicly warned other orthopedists about cup failures his patients were experiencing.”84 Dorr and three colleagues later presented their experiences with the Durom Cup failures at the February 2009 meeting of the American Academy of Orthopedic Surgeons.85 The surgeons maintained that the cup loosened in 25 percent of patients, leading to revision surgeries in 20 percent. In their abstract, the surgeons attributed the failures to “the cup geometry and sharp peripheral fins which result in peripheral fixation without contact of fixation surface to acetabular bone in some hips. Because we cannot predict in which patients this will occur we no longer use the Durom cup.”86 Zimmer, however, concluded, after an “in-depth investigation,” that the Durom cup design was not the problem.87
Zimmer said that it studied the patient outcomes at 12 clinical sites representing the highest volumes of implants using the Durom Cup, and estimated the likely revision rate of 1.5 percent. “No evidence of a defect in the materials, manufacture, or design of the implant has been found.”88 Instead, Zimmer said, “the instructions for use/surgical technique instructions were inadequate.”89 Zimmer vowed to launch a campaign to offer “additional surgical technique instructions and training are necessary in the United States, and we strongly recommend that U.S. surgeons stop implanting the Durom Cup until receiving such training.”90 It also promised to update the labeling to provide more detailed surgeon instructions. Meanwhile the Durom cup would continue to be marketed “without interruption” abroad.91
Three weeks later, Zimmer announced that it had revised its surgical training and instructions and would resume selling the Durom cup to surgeons who completed the new courses, offered in a variety of settings.92 Besieged by lawsuits relating to Zimmer Durom Cup hip failures, the company set aside millions to pay legal claims.93 “We have paid approximately $45 million and $25 million in 2010 and 2009, respectively, related to Durom Cup product liability claims. We estimate the remaining liability for Durom Cup claims as of December 31, 2010, is $132.8 million.”94 In 2010, surgeons from the University of Wisconsin Hospital and Clinics’ Department of Orthopaedics and Rehabilitation, reported an 11.1 percent failure rate – defined by persistent moderate or severe groin pain or revision – among its patients implanted with the Durom cup, compared to a .002 rate for those implanted with components from Zimmer’s Trilogy acetabular component.95 “We could not identify any patient/surgical-related factors predictive of failure,” the researchers concluded.96
FDA Approval and Surveillance Process
In 1976, public outcry over failed medical devices, (most prominently, the deaths and infertility caused by the Dalkon shield, a contraceptive intrauterine device), prompted Congress to amend the Food, Drug and Cosmetics Act. The Medical Device Amendments granted the FDA authority to regulate medical devices, and provided two pathways to market.97 The more rigorous schema, pre-market approval (PMA), requires that the manufacturer to prove that the device is safe and effective. Pre-market Notification (PMN), known popularly by the sub-section of the regulation 510(k), requires that the device “is at least as safe and effective, that is, substantially equivalent, to a legally marketed device.”98 The FDA sorted new and existing devices into three basic categories: pre-amendment devices, post-amendment devices and substantially equivalent devices – new devices based on pre-amendment devices or approved post-amendment devices.
At the same time, the agency classified devices according to their level of risk to human health, design complexity and use. Class I devices, such as tongue depressors and crutches, posed the lowest risk. Class II devices included those that posed an intermediate risk, such as contact lens solution and electrocardiographs. Class III devices encompassed those that carried the highest risk and supported or sustained human life, or prevented the impairment of health, such as HIV diagnostic tests and implantable devices, such as pacemakers. Pre-Market Approvals invoke the most stringent FDA “scientific and regulatory review,” which includes an in-depth scientific and quality system review; a review and recommendation by an advisory committee; and final deliberations by the FDS before a decision is made.”99 Manufacturers seeking a PMA must submit data from a clinical controlled trial and be subject to a manufacturing inspection. Most Class III devices are subject to the PMA process.
Pre-Market Notification or 510k was established to streamline the process for new medical devices that were “substantially equivalent” to devices – called “predicates” in the approval process – that were already on the market by 1976. Many new versions of older Class III devices, grandfathered by the Medical Device Amendments, are regulated under the 510k process until the FDA can provide an effective date for the device’s PMA. In 2002, the aggressive deregulation of the George W. Bush presidency resulted in a further loosening of the 510k process. The FDA was directed to take “the least burdensome approach to medical device regulation,”100 and the definition of “substantially equivalent” now included products made using different materials and mechanics than the predicate device. Predicates no longer had to be in the marketplace in 1976; any device already cleared – either by 510k or PMA – applied. In result, 510k became the de facto route to FDA approval, with 99 percent of all devices approved under this scheme. The regulations now allowed patients to be exposed to devices that had never been subjected to clinical trials, testing or any standards.101Today, the FDA considers a device marketable under 510k if the new device:
- Has the same intended use as the predicate; and has the same technological characteristics as the predicate; or has the same intended use as the predicate; and has different technological characteristics and the information submitted to FDA
- Does not raise new questions of safety and effectiveness; and demonstrates that the device is at least as safe and effective as the legally marketed device.102
Manufacturers can claim substantial equivalence based on “intended use, design, energy used or delivered, materials, chemical composition, manufacturing process, performance, safety, effectiveness, labeling, biocompatibility, standards, and other characteristics, as applicable.”103 The FDA gives wide latitude to what may be considered substantially equivalent. A medical device manufacturer could submit a 510k if the device has a different intended use than the predicate. It can seek approval under 510k “if there is a change or modification of a legally marketed device and that change could significantly affect its safety or effectiveness.” The 510k process is much quicker and cheaper than a pre-market approval. The FDA has 90 days to assess a manufacturer’s declaration of substantial equivalence.
Once approved, the device may be marketed to the public. Many clinicians, public health advocates have been highly critical of the 510k process, and have documented the increased risk of patient harm created by the release of problematic health devices. This April, the Senate Special Committee on Aging held a hearing on the safety of medical devices.104 In addition to Korgaokar, the Committee heard from the Government Accountability Office, which is in the midst of an ongoing investigation on the FDA’s medical device approval process, post-market monitoring and recalls, and Dr. Diana Zuckerman, who recently published a study of medical device recalls.
The committee also heard from manufacturers’ representatives who expressed fears that the FDA process was slowing the pace of medical device innovation. The majority of the witnesses, however, raised concerns that the 501K process is not effective in ensuring the safety of new devices. U.S. Senator Herb Kohl, chairman of the Special Committee on Aging, vowed to advocate for further reforms, and “push forward with an effort to classify high-risk devices now defaulting through the agency’s ‘fast track’ approval system.”105 “FDA has had over 20 years to tackle these high risk devices,” Kohl said. “As we have seen with the Johnson & Johnson hip implant today, it’s high time to protect patient safety and correctly classify these devices.”106 In February, The Archives of Internal Medicine published a five-year study of recalls for devices that FDA determined could cause “serious health problems or death,” and found that nearly three-quarters were approved via the 510K process.107
Of a total of 113 such recalls, from 2005 through 2009, 21 were PMA-approved; 80 entered the stream of commerce via 510k and 8 were exempt from any FDA regulations. The authors concluded: “Medical devices cleared through the less rigorous 510(k) pathway comprise more than two-thirds of the products that are recalled by the FDA because they could seriously harm patients or result in death. When devices that were intentionally exempt from any FDA review were added to the 510(k) devices, they comprise more than 3 out of 4 of the high-risk recalls during the last 5 years. Thus, the standards used to determine whether a medical device is a high-risk or life-sustaining product prior to approval are clearly very different from the standards used to recall a medical device as life threatening.
Our findings reveal critical flaws in the current FDA device review system and its implementation that will require either congressional action or major changes in regulatory policy.108 In the summer of 2009, the General Accounting Office (GAO) issued a report on the FDA’s medical devices review processes. It found that between 2003 and 2007, the FDA reviewed 342 submissions for Class III devices through the 510(k) process, clearing 67 percent for marketing – even though Congress envisioned that all Class III devices would be subject to the more rigorous pre-market approval process.109 It recommended that the “FDA expeditiously take steps to issue regulations for each class III device type currently allowed to enter the market through the 510(k) process, including (1) reclassifying each device type into a lower class or requiring it to remain in class III and (2) for those device types remaining in class III, requiring approval for marketing through the PMA process.”
By September 2009, the FDA’s Center for Devices and Radiological Health convened two working groups to address the criticisms directed at the 510k process.110 Industry complained that it was too arbitrary and unpredictable, and that the CDRH reviewers “had become less responsive and risk adverse.”111 Patients, professional healthcare groups and insurers countered that 510K didn’t do enough to assure safety or effectiveness. The 510(k) Working Group and the Task Force on the Utilization of Science in Regulatory Decision Making. CDRH staff had its own problems with 510k. They asserted that a burgeoning workload, along with the increasing complexity of devices and the poor quality of 510k submissions made it difficult to make informed decisions. In August 2010, the two groups issued 55 recommendations, and in January, the FDA announced a plan to begin implementation.112
Among the recommended changes was an explicit requirement that all 510(k) submitters “provide a summary of all scientific information known or that the submitter should reasonably know regarding the safety and effectiveness of the device under review;” that CDRH develop a guidance document that clarifies when a device should not be used as a predicate and promulgating a new regulation clarifying “the circumstances under which the center would exercise its authority to rescind a 510(k) clearance to remove an unsafe device from the market and preclude its use as a predicate.”11
Hip Arthroplasty and FDA Approval and Surveillance
Hip implants should be, by FDA definition, designated as a Class III device. (Under the Medical Device amendments, all Class III devices will require a PMA. However, if a Class III device was in existence before 1976, the manufacturer can continue to market the device until the FDA publishes a regulation calling for PMA submissions.)114 Nonetheless, in 1998, to streamline the pre-market approval process, the FDA re-classified hip arthroplasty as Class II Devices.115 Regardless of risk classification, the vast majority of hip replacement devices in use have been brought to market under 510K. According to a 2008 study published in The Open Rheumatology Journal, in 1976, the few hip replacement devices already on the market were grandfathered under the MDA.116
The researchers looked at all total hip replacement approvals granted between 1976 and 1995, and found that 701 devices produced by 74 manufacturers were brought to market via the 510k process, compared to 34 such devices, from just four manufacturers, subjected to the more rigorous PMA process. Up until 1991, 297 prosthetic hip devices were approved. The last five years of the study period, all 404 devices – representing 60 percent of the all approvals in two decades – were approved as substantially equivalent devices. “By satisfying this requirement, manufacturers were not required to provide clinical data on the safety and effectiveness of their device,” the authors wrote.117 These researchers also found that the FDA surveillance system did a poor job of following the failure rate of these implants.
The FDA’s Mandatory Device Reporting requires that any device causing a serious injury, defined as “all events necessitating a return to the operating room related to a medical device failure.” By this measure, they assert, almost all revision arthroplasties should be reported. The researchers mined two independent sources of data for hip replacement revision surgeries performed in 1993 – the MDR and the National Hospital Discharge Survey and estimated that only three percent of all such revisions were reported to the FDA; only 15 percent of the approved hip replacement devices published data on outcomes. In addition to the technical literature documenting the laxness of the approval and surveillance process, the Department of Justice has investigated and obtained multi-million-dollar settlements from four of the five major Total Hip Replacement manufacturers.118
The investigation, which began in 2005, culminated in charges that the manufacturers used consulting agreements with orthopedic surgeons to induce the purchase of their devices Zimmer, Inc., Depuy Orthopaedics, Inc., Biomet Inc., and Smith & Nephew, Inc. for $311 million from four manufacturers of hip and knee surgical implant products–to settle claims that from at least 2002 through 2006 these companies.119 The government’s investigation revealed that the firms paid surgeons hundreds of thousands of dollars a year for consulting contracts and lavished them with trips and other expensive perquisites in exchange for using the companies’ products exclusively. In addition to the civil settlements, the four companies executed deferred prosecution agreements requiring new corporate compliance procedures and the appointment of federal monitors to review their compliance with these procedures.120
History of Total Hip Arthroplasty
Surgeons pioneered Metal-on-Metal hip replacement in a series of 26 operations performed between 1956 and 1960. The revision rate was about 30-40 percent, and failures, mostly due to impingement of the acetabular cup on the femoral neck resulting in notching and wear debris, were accompanied by “varying amounts of sludge containing many metal particles.”121 The 1960′s ushered in a wave of Metal-on-Metal Total Hip Replacements made of cast Cobalt-Chromium-Alloy or stainless steel. Initial products included: McKee-Farrar (Wiles and McKee; UK); Ring (UK); Mueller-Huggler (Switzerland); Sivash (Soviet Union); and Stanmore (Scales and Wilson; England). These early designs were relatively simple in design and quality. Loosening rates were high and researchers were concerned about the biological reaction to the alloys, known as metal sensitivity. For example, the McKee-Farrar design suffered from high loosening rates because of equatorial bearing and impingement of the femoral neck against the rim of the acetabular component.122
When the first-generation Metal-on-Metal implants were revised, doctors discovered a variety of adverse tissue reactions. Macrophages – white blood cells that ingest foreign matter as part of the body’s defense system – consumed the metal wear particles and died, potentially compromising the surrounding tissue and immune system. Doctors also discovered osteolysis (the dissolution of bone tissue) and many tissues were stained with black debris from the metal particulate. These adverse effects were mostly associated with impingement or loose components rather than with functioning implants. Nonetheless, some evaluations show that some of these designs had low wear rates and long term success despite their designs.
Sir John Charnley, regarded as the pioneer of modern hip replacement surgery, advanced the state of the art by introducing Ultra High Molecular Weight Polyethylene (UHMWPE) to arthroplasty in the 1960′s. This material has been used for more than 40 years and is still the most frequently used bearing surface in all total joint replacements, including hip, knee, and shoulder implants.123 In the early 1960′s, Charnley, a British orthopedic surgeon, developed a low-friction arthroplasty consisting of a femoral head and an all-polyethylene cup. Since then, metal-on-polyethylene with a metal femoral head and polyethylene acetabular cup is the most frequently implanted device. (ceramic-on-polyethylene devices are also popular and boast lower wear rates than metal-on-polyethylene.124) By 1975, surgeons concerned about the adverse effects of metal particulate began to phase-out Metal-on-Metal systems, and metal-on-polyethylene became the implant of choice.
By the end of that decade, there was a widespread perception that the metal-on-polyethylene systems gave better clinical results,125 126 and this became the preferred route for total hip replacements throughout the 1980′s. The soft-on-hard combinations develop wear on the plastic part of the device – the result of the high activity level of the patient, thin polyethylene liners, excessive abduction (laterally) of the acetabular cup or the use of modular uncemented cups.127 128
The generated debris can cause an adverse reaction in the tissue, so wear remains the biggest problem of this type of total hip arthroplasty. Eventually, the polyethylene wear particles can cause osteolysis (destruction of the bone) around that implant and lead to the loosening of the cup, the primary reason for revision in the long term total hip arthroplasty, especially in young and active patients.130 131 132 133 134 (Despite the problems of polyethylene wear, some surgeons still prefer polyethylene liners because they provide the patient with better shock absorption and are more forgiving when misaligned. A less expensive material, polyethylene does not release metal wear debris into the bloodstream, squeak or fracture easily.135 136 137 Although high-contact stresses in the polymer due to very thin liners or edge loading may lead to component fracture.138) On a quest to reduce the wear and osteolysis associated with polyethylene components, French physician, Pierre Boutin, pioneered the use of ceramic-on-ceramic bearings for total hip arthroplasty in the 1970′s.139 Some surgeons favor the use of ceramic femoral head and polyethylene cup as an alternative to a metal-on-polyethylene device, due to the excellent mechanical qualities and because the smooth surface finish reduces the problems of wear.
Compared to metallic heads, ceramic femoral heads paired with polyethylene cups have generally been associated with as much as a 50 percent reduction wear rate compared to metal-on-polyethylene,140 but still more than Metal-on-Metal bearings.141 Ceramic-on-ceramic hip implants offer several advantages. First, the opposing surfaces (the acetabular cup and the femoral head) can be consistently manufactured to minimal surface roughness and controlled radial clearances. This, combined with very hard scratch-resistant ceramic materials, provides an excellent surface for lubrication.142 143 In addition, alumina (the most commonly used ceramic material) wear debris generated by a ceramic-on-ceramic implant is chemically inert and does not have a toxic effect on the body. The damage to surrounding tissues is less, adverse cellular reactions and macrophage response are considerably fewer compared to the body’s reactions to metallic or polyethylene debris.144 145
Ceramic-on-ceramic implants, however, also have their downside. Some patients complain of post-operative squeaking related to the orientation of the acetabular component.146 The alumina-on-alumina bearings are sensitive to the position of the cup and edge-loading, increasing their propensity to chip and fracture.147 148 149 Finally, ceramic-on-ceramic implants are relatively expensive and poor implant position can increase the wear rate.150 In 1988, Bernard C. Weber,151 a Swiss orthopedic surgeon, revived the use of metal on metal hip implants.152 Securing the support of device manufacturer Sulzer Orthopedics, Weber developed the Metasul Metal-on-Metal bearing, which is still being used today. The problem of early component loosing seen in the first generation Metal-on-Metal implants was allegedly corrected by improved component design (tolerance and clearance), improved metallurgy, better manufacturing quality, improved bearing geometry (sphericity and clearance), and a smoother surface finish to promote natural lubrication. Improved cobalt-chromium alloys such as wrought or cast, high carbon or lower carbon alloys were developed. These materials significantly improved laboratory wear rates compared to initial Metal-on-Metal and especially metal-on-polyethylene bearing surfaces. Others followed suit. In the 1990′s, many patients were implanted with the Metasul hip system. One study that followed patients’ progress five years after surgery found a very low rate of loosening of 0 to 1.3 percent.153 154 155 156 157
Conclusions
While total hip arthroplasty is a well-established and often successful implant procedure, many new designs, using Metal-on-Metal components, are prone to early failure and introduce significant health hazards in patients. The recipients of defective hip devices are exposed to an array adverse effects ranging from bone loss to tissue death to poisonous levels of metal wear particles, and are subject to pain and unnecessary and debilitating second surgeries. Manufacturers have taken advantage of the regulatory agency’s attempts to streamline the approval process, by claiming that new devices are simply a variation of an existing hip arthroplasty device. This has allowed too many devices that are critical to a patient’s health and well being to be implanted into patients without proving efficacy or safety.
The U.S. Food and Drug Administration’s attempts to reform the medical device approval process have come too late to prevent defective hip arthroplasty designs from coming to the marketplace and being implanted at great risk to consumers. While some have been recalled, others, such as DePuy’s Pinnacle Metal-on-Metal hip system have not. In addition to reforms the FDA is currently implementing, the agency should immediately implement a post-market surveillance program to uncover hip replacement systems with revision rates outside of the norm and press those manufacturers to recall those devices immediately.
References:
- Testimony of Katherine Korgaokar; A Delicate Balance: FDA and the Reform of the Medical Device Approval Process; Senate Committee on Aging; April 13, 2011
- Testimony of Katherine Korgaokar; A Delicate Balance: FDA and the Reform of the Medical Device Approval Process; Senate Committee on Aging; April 13, 2011
- Testimony of Katherine Korgaokar; A Delicate Balance: FDA and the Reform of the Medical Device Approval Process; Senate Committee on Aging; April 13, 2011
- A Comparative Joint Simulator Study Of The Wear Of Metal-on-Metal And Alternative Material Combinations. In Hip Replacements; A A J Goldsmith; D Dowson; G H Isaac; J G Lancaster; Proceedings Institution of Mechanical Engineers; Vol 214 Part H; 1999
- Orthopedic Surgery Advances; Ron Stoker; Infection Prevention in the OR; HealthVIE.com; January 2011
- Current Concepts on Metal on Metal Resurfacing; Ian C. Clarke, et al; Orthopedic Clinics of North America; 2005
- Current Concepts on Metal on Metal Resurfacing; Ian C. Clarke, et al; Orthopedic Clinics of North America; 2005
- Advancing High Stability and Low Wear; Brochure; DePuy Orthopaedics; 2008
- Benefits of Metal On Metal; Brochure; DePuy Orthopaedics; 2008
- Wear and Lubrication of Metal-on-Metal Hip Implants; Frank W. Chan, PhD; J.Dennis Bobyn, PhD.; John B. Medley, PhD; Jan J. Krygier, CET and Michael Tanzer, MD; Clinical Orthopaedics and Related Research, 1999
- A comparative joint simulator study of the wear of Metal-on-Metal and alternative material combinations in hip replacements; A.A.J. Goldsmith; D. Dowson; G.H. Isaac and J.G. Lancaster; Proc Instn Mech Engrs, 1999
- Metal-on-Metal bearing in hip prosthesis generates 100-fold less wear debris than metalon-polyethylene; H. Lucas Anissian; Andre Stark; Allen Gustafson; Victoria Good and Ian C Clarke; Acta Orthop Scand, 1999
- Quantitative analysis of wear debris from Mom Hip prostheses tested in a physiological hip joint simulator. Firkins. et al, Proceedings of the 45th Annual Meeting of the ORS, 1999
- Quantitative analysis of the wear and wear debris from low and high carbon content cobalt chrome alloys used in MoM THR. Tipper, et al. J Mater SciMater Med, 1999
- 15 The Clinical Performance of Metal on Metal as an Articulation Surface in Total Hip Replacement, Long; The Iowa Orthopaedic Journal, 2005
- 16 Cementless Metal-on-Metal Hip Arthroplasty in Patients Less Than 50 Years of Age; H. Migaud, A. Jobin, C. Chantelot, F. Giraud, P. Laffargue, A. Duquennoy; The Journal of Arthroplasty, 2004
- 17 Metal-on-Metal Versus Metal-on-Polyethylene Bearings in Total Hip Arthroplasty; D. Naudie, C. P. Roeder, J. Parvizi, D. J. Berry, S. Eggli, A. Busato. The Journal of Arthroplasty, 2004
- Three- to Six-Year Results With the Ultima Metal-on-Metal Hip Articulation for Primary Total Hip Arthroplasty; Michael Jacobs, Robert Gorab, David Mattingly, Lorence Trick, Carleton Southworth. The Journal of Arthroplasty, 2004
- http://www.fda.gov
- Australian National Joint Registry Annual Reports 2008, 2009, 2010
- Australian National Joint Registry Annual Reports 2008, 2009, 2010
- Long-duration MoM THA with low wear of the articulating surfaces. Thomas P. Schmalzried, Paul C. Peters, Brian T. Maurer, Charles R. Bragdon, William H. Harris. J Arthroplasty, 1996
- Complications After Metal-on-Metal Hip Resurfacing Arthroplasty; Harlan C. Amstutz; Michel J. Le Duff; Patricia A. Campbell; Lauren E. Wisk and Karren M. Takamura, Orthopedic Clinics of North America, 2010
- Metal-Metal Bearing Surfaces in Hip Arthroplasty. Schmalzried. Orthopedics 2009.
- Mayo Medical Laboratories interpretive handbook: interpretive data for diagnostic laboratory tests. Rochester, NM: The Laboratories, Leavelle, Ed. 2001
- Arthroprosthetic cobaltism: identification of the at-risk patient. Tower, et al., Alaska Medicine, 2010
- Trace Metals in the environment. V6: Cobalt. An appraisal of environmental exposure. Smith, et al. Ann Arbor, MI: Ann Arbor Science, 1981
- Arthroprosthetic Cobaltism: Neurological and Cardiac Manifestations in Two Patients with Metal-on-Metal Arthroplasty; Stephen S. Tower, MD; JBJS Am 2010
- 2010 MHRA COM Report
- Early failure of MoM bearings in hip resurfacing and large-diameter THR: a consequence of excess wear. Langton, et al. JBJS Br, 2010
- Characterization of the running-in period in THRA: an in vivo and in vitro metal ion analysis. Heisel, et al. JBJS Am, 2008
- Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after MoM hip resurfacing. Hart, et al. Hip Int, 2008
- A hip joint simulator study of the performance of MoM joints. Part II Design. Dowson, et al. J Arthroplasty 2004
- Influence of the clearance on in-vitro tribology of large diameter MoM articulations pertaining to resurfacing hip implants. Rieker, et al. Orthop Clin NA, 2005
- Early clinical failure of the Birmingham MoM hip resurfacing is associated with metallosis and soft-tissue necrosis. Ollivere, et al. JBJS Br, 2009
- Correlation between inclination of the acetabular component and metal ion levels in MoM hip resurfacing replacement. De Haan, et al. JBJS Br, 2008
- The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. Langton, et al. JBJS Br, 2008
- Chromium and Cobalt ion release following the Durom high carbon content, forged MoM surface replacement of the hip. Vendittoli,et al. JBJS Br,. 2007
- Clinical and radiographic results of MoM hip resurfacing with minimum ten year follow up. Amstutz, et al. JBJS Am, 2010
- Complications After Metal-on-Metal Hip Resurfacing Arthroplasty. Harlan C. Amstutz; Michel J. Le Duff; Patricia A. Campbell; Lauren E. Wisk and Karren M. Takamura. Orthopedic Clinics of North America, 2011
- Tribological analysis of failed resurfacing hip prostheses and comparison with clinical data. T.J Joyce; D.J. Langton; S.S. Jameson and A.V.F Nargo. Proc. IMechE, 2009.
- The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. D.J. Langton; S.S. Jameson; T.J. Joyce; J. Webb and A.V.F. Journal of Bone and Joint Surgery, 2008
- Ceramic-On Metal Bearings In Total Hip Replacement. Isaac, Brockett, Breckon, van der Jagt, Williams, Hardaker, Fisher, Schepers. JBJS Br, 2009.
- Complications After Metal-on-Metal Hip Resurfacing Arthroplasty. Harlan C. Amstutz; Michel J. Le Duff; Patricia A. Campbell; Lauren E. Wisk and Karren M. Takamura. Orthopedic Clinics of North America, 2011
- Correlation between inclination of the acetabular component and metal ion levels in MoM hip resurfacing replacement. De Haan, et al. JBJS Br 2008; 90(10):1291
- Risk factors for inflammatory pseudotumour formation following hip resurfacing. Glyn-Jones, JBJS Br 2009;91(12):1566
- Complications After Metal-on-Metal Hip Resurfacing Arthroplasty. Harlan C. Amstutz; Michel J. Le Duff; Patricia A. Campbell; Lauren E. Wisk and Karren M. Takamura. Orthopedic Clinics of North America, 2011.
- Hip Resurfacing arthroplasty. Mont, et. al., J Am Acad Orhtop Surg 2006; 14:454.
- DO_Advancing_High_Stability_and_Low_Wear_Brochure_0612-17-508r1
- Benefits of Metal On Metal. DuPuy Brochure
- High Cup Angle and Microseparation Increase the Wear of Hip Surface Replacements. Ian J. Leslie PhD; Sophie Williams PhD; Graham Isaac PhD; Eileen Ingham PhD and John Fisher PhD. Clin Orthop Relat Res, 2009.
- Tribological analysis of failed resurfacing hip prostheses and comparison with clinical data. T.J Joyce; D.J. Langton; S.S. Jameson and A.V.F Nargo. Proc. IMechE Vol. 223 Part J: J. Engineering Tribology, 2009.
- Early failure of MoM bearings in hip resurfacing and large-diameter THR: a consequence of excess wear. Langton, et al. JBJS Br 2010:92(1):38
- Characterization of the running-in period in THRA: an in vivo and in vitro metal ion analysis. Heisel, et al. JBJS Am 2008:90 (Suppl 3): 125.
- Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after MoM hip resurfacing. Hart, et al. Hip Int 2008;18(3):212.
- A hip joint simulator study of the performance of MoM joints. Part II Design. Dowson, et al. J Arthroplasty 2004; 19 (8 Suppl 3): 124-30.
- Influence of the clearance on in-vitro tribology of large diameter MoM articulations pertaining to resurfacing hip implants. Rieker, et al. Orthop Clin NA 2005;36 (2):135.
- 58 Correlation between inclination of the acetabular component and metal ion levels in MoM hip resurfacing replacement. De Haan, et al. JBJS Br 2008;90(10):1291.
- 59 The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. Langton, et al. JBJS Br. 2008;90(9):1143.
- Chromium and Cobalt ion release following the Durom high carbon content, forged MoM surface replacement of the hip. Vendittoli, et al. JBJS Br. 2007;89(4):441.
- Complications After Metal-on-Metal Hip Resurfacing Arthroplasty. Harlan C. Amstutz; Michel J. Le Duff; Patricia A. Campbell; Lauren E. Wisk and Karren M. Takamura. Orthopedic Clinics of North America, 2011.
- Tribological analysis of failed resurfacing hip prostheses and comparison with clinical data. T.J Joyce; D.J. Langton; S.S. Jameson and A.V.F Nargo. Proc. IMechE Vol. 223 Part J: J. Engineering Tribology, 2009.
- The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. D.J. Langton; S.S. Jameson; T.J. Joyce; J. Webb and A.V.F. Nargol. The Journal of Bone and Joint Surgery, 2008
- Ceramic-On Metal Bearings In Total Hip Replacement. Isaac, Brockett, Breckon, van der Jagt, Williams, Hardaker, Fisher, Schepers. JBJS Br, 2009.
- The Kaiser Permanente National Joint Replacement Registry; web page; http://xnet.kp.org/permanentejournal/sum08/joint-replacement.html; accessed May 3, 2011
- The Kaiser Permanente National Joint Replacement Registry; web page; http://xnet.kp.org/permanentejournal/sum08/joint-replacement.html; accessed May 3, 2011
- Surgeon Vrs. Knee-Maker: Who’s Rejecting Whom?; Barry Meier; The New York Times; June 10, 2010.
- Manufacturer and User Facility Device Experience; U.S. Food and Drug Administration; http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm; accessed May 1, 2011
- FDA K040627; 510K Summary; DePuy Orthopaedics; August 5, 2005
- FDA K073413; 510K Summary; DePuy Orthopaedics; January 30, 2008
- MAUDE Report 1836490
- MAUDE Report 1932627
- MAUDE Report 1937943
- Minutes of the Committee on the Safety of Devices Expert Advisory Group on metal wear debris from hip implants; Meeting minutes; October 23, 2006
- Minutes of the Committee on the Safety of Devices Expert Advisory Group on metal wear debris from hip implants; Meeting minutes; October 23, 2006
- Statement On Biological Effects Of Wear Debris Generated From Metal On Metal Bearing Surfaces: Evidence For Genotoxicity; Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment; July 2006
- Statement On Biological Effects Of Wear Debris Generated From Metal On Metal Bearing Surfaces: Evidence For Genotoxicity; Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment; July 2006
- Biological Effects Of Metal Wear Debris Generated From Hip Implants: Genotoxicity; Medicines and Healthcare products Regulatory Agency; July 2006
- DePuy April 15, 2011
- Large Diameter Metal on Metal Bearing Total Hip Replacements; Statement, John Hodgkinson; John Skinner; Peter Kay; British Orthopaedic Association; March 9, 2011
- Large Diameter Metal on Metal Bearing Total Hip Replacements; Statement, John Hodgkinson; John Skinner; Peter Kay; British Orthopaedic Association; March 9, 2011
- Large Diameter Metal on Metal Bearing Total Hip Replacements; Statement, John Hodgkinson; John Skinner; Peter Kay; British Orthopaedic Association; March 9, 2011
- Large Diameter Metal on Metal Bearing Total Hip Replacements; Statement, John Hodgkinson; John Skinner; Peter Kay; British Orthopaedic Association; March 9, 2011
- Complaints Undermine Hip Device; Barry Meier; New York Times; July 24, 2008
- Metal-on-Metal total hip arthroplasty using Durom cup; Lawrence Dorr, et al.; 2009 Annual Meeting Poster Presentations; American Academy of Orthopaedic Surgeons; February
- Metal-on-Metal total hip arthroplasty using Durom cup; Lawrence Dorr, et al.; 2009 Annual Meeting Poster Presentations; American Academy of Orthopaedic Surgeons; February
- Urgent Device Correction; Cheryl Blanchard; Letter to surgeons; DePuy Orthopaedics; July 22, 2008
- Urgent Device Correction; Cheryl Blanchard; Letter to surgeons; DePuy Orthopaedics; July 22, 2008
- Urgent Device Correction; Cheryl Blanchard; Letter to surgeons; DePuy Orthopaedics; July 22, 2008
- Urgent Device Correction; Cheryl Blanchard; Letter to surgeons; DePuy Orthopaedics; July 22, 2008
- Urgent Device Correction; Cheryl Blanchard; Letter to surgeons; DePuy Orthopaedics; July 22, 2008
- Urgent Device Correction-Update; Cheryl Blanchard; Letter to surgeons; DePuy Orthopaedics; August 16, 2008
- Zimmer Holdings, Inc.; Form 10K Filing; Dec. 31, 2010
- Zimmer Holdings, Inc.; Form 10K Filing; Dec. 31, 2010
- Large-Head Metal-on-Metal Total Hip Arthroplasty Using the Durom Acetabular Component at Minimum 1-Year Interval; Richard L. Illgen II; John P. Heiner; Matthew W. Squire; David N. Conrad; The Journal of Arthroplasty Vol. 25 No. 6 Suppl. 1; 2010
- Large-Head Metal-on-Metal Total Hip Arthroplasty Using the Durom Acetabular Component at Minimum 1-Year Interval; Richard L. Illgen II; John P. Heiner; Matthew W. Squire; David N. Conrad; The Journal of Arthroplasty Vol. 25 No. 6 Suppl. 1; 2010
- Medical Devices Amendment; PL 94-295; May 28, 1976
- Premarket Notification 510k; accessed from http://www.fda.gov; Food and Drug Administration; 20110408
- Eventually all Class III devices will require a PMA. However, preamendment Class III devices require a PMA only after FDA publishes a regulation calling for PMA submissions. The preamendment devices must have a PMA filed for the device by the effective date published in the regulation in order to continue marketing the device.
- Medical Device Recalls and the FDA Approval Process; Diana M. Zuckerman, PhD; Paul Brown, BS; Steven E. Nissen, MD; Archives of Internal Medicine; February 14, 2011
- Medical Device Recalls and the FDA Approval Process; Diana M. Zuckerman, PhD; Paul Brown, BS; Steven E. Nissen, MD; Archives of Internal Medicine; February 14, 2011
- U.S. Food and Drug Administration; April 14, 2011
- U.S. Food and Drug Administration; April 14, 2011
- FDA’S Medical Device Review Scrutinized At Senate Hearing; Press release; Special Committee on Aging; April 13, 2011
- FDA’S Medical Device Review Scrutinized At Senate Hearing; Press release; Special Committee on Aging; April 13, 2011
- FDA’S Medical Device Review Scrutinized At Senate Hearing; Press release; Special Committee on Aging; April 13, 2011
- Medical Device Recalls and the FDA Approval Process; Diana M. Zuckerman, PhD; Paul Brown, BS; Steven E. Nissen, MD; Archives of Internal Medicine; February 14, 2011
- Medical Device Recalls and the FDA Approval Process; Diana M. Zuckerman, PhD; Paul Brown, BS; Steven E. Nissen, MD; Archives of Internal Medicine; February 14, 2011
- Medical Devices: FDA Should Take Steps to Ensure That High-Risk Device Types Are Approved through the Most Stringent Premarket Review Process; Government Accounting Office; January 2009
- FDA to improve most common review path for medical devices; Press release; U.S. Food and Drug Administration; January 19, 2011
- 510(K) and Science Report Recommendations; August 2010
- FDA to improve most common review path for medical devices; Press release; U.S. Food and Drug Administration; January 19, 2011
- FDA Issues Assessments of the 510(k) Program and Use of Science in Decision-Making; Press release; U.S. Food and Drug Administration; August 4, 2010
- U.S. Food and Drug Administration; April 14, 2011
- Improving the Postmarket Surveillance of Total Joint Arthroplasty Devices; Nizar N Mahomed, Khalid Syed, Clement B. Sledge, Troyen A Brennan, and Matthew H Liang; The open Rheumatology Journal; February 25, 2008
- Improving the Postmarket Surveillance of Total Joint Arthroplasty Devices; Nizar N Mahomed, Khalid Syed, Clement B. Sledge, Troyen A Brennan, and Matthew H Liang; The open Rheumatology Journal; February 25, 2008
- Improving the Postmarket Surveillance of Total Joint Arthroplasty Devices; Nizar N Mahomed, Khalid Syed, Clement B. Sledge, Troyen A Brennan, and Matthew H Liang; The open Rheumatology Journal; February 25, 2008
- Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring; Christopher J. Christie; United States Department of Justice; September 27, 2007
- Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring; Christopher J. Christie; United States Department of Justice; September 27, 2007
- Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring; Christopher J. Christie; United States Department of Justice; September 27, 2007
- Current Concepts on Metal on Metal Resurfacing; Ian C. Clarke, et al; Orthopedic Clinics of North America; 2005
- Long Duration Metal-on-Metal Total Hip Arthroplasties; Thomas P. Schmalzried; Paul C. Peters; Brian T. Maurer; Charles R. Bragdon; William H. Harris; The Journal of Arthroplasty Vol. 11 No. 3; 1996
- Arthroplasty of the Hip-a new operation. Charnley, et al. Lancet 1:1129, 1961.
- Bearings of the Future for Total Hip Arthroplasty. Michael T. Manley, FRSA, PhD and Kate Sutton, MA, ELS. The Journal of Arthroplasty, 2008
- THR by low-friction arthroplasty. Charnley, et. al., Clin Orhtop 1970; 72:7.
- The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. Charnley, et. al. JBJS Br 1972;54B:61
- 3D PE Wear of a Press-Fit Ti prosthesis-Factors influencing generation of PE Debris. Devane, et. al., J Arthroplast 12:256, 1997.
- Effect of acetabular modularity on PE Wear and Osteolysis in THA. Young, et.al. JBJS Am 84: 58, 2002.
- Wear and periprosthetic osteolysis: the problem. Harris, et. al. Clin Orthop 393:66-70. 2001
- Fifteen years’ experience with Charnley low-friction arthroplasty. Hartofilakidis, et. al. Clin Orhtop 246:48. 1989.
- Early and Late Loosening of the acetabular cup after low-friction arthroplasty. Garcia-Cimbrelo, et. al. JBJS 74:1119. 1992
- Low-friction arthroplasty in patients younger than 40 years old: 20-25 year results. Garcia-Cimbrelo, et. al. J Arthroplast 15: 825, 2000
- THA for primary osteoarthrosis in younger patients in the Finnish arthroplasty register. Eskelinen, et. al. Acta Orthop 76: 28, 2005
- Long-term results of use of the anatomic medullary locking prosthesis in THA. Engh, et. al. JBJS Am 79: 177, 1997.
- Levels of metal ions after small and large-diameter MOM Hip Arthroplasty. Clarke, et. al. JBJS Br 85:913, 2003.
- Preliminary observations on possible premalignant changes in bone marrow adjacent to worn total hip arthroplasty implants. Case, et. al. Clin Orthop 329;s269, 1996.
- Squeaking in CoC hips-the importance of acetabular component orientation. Walter, et. al. J Arthroplast 22:496, 2007
- Bearings of the Future for Total Hip Arthroplasty. Michael T. Manley, FRSA, PhD and Kate Sutton, MA, ELS. The Journal of Arthroplasty, 2008
- Total arthroplasty of the hip by fitted aluminum prosthesis. Experimental study and 1st clinical applications. Boutin, et.al. Rev Chir Orthop Reparatrice Appar Mot 1972, 58
- Bearing Surface Options for the THR in young patients. Heisel, et. al. JBJS Am 85:1366, 2003.
- A comparative joint simulator study of the wear of the wear of Metal-on-Metal and alternative material combinations in hip replacements. Goldsmith, Dowson, Isacc, Lancaster. Proc Instn Meck Engrs, V 214, Part, H, 1999.
- A full numerical analysis of hydrodynamic lubrication in artificial hip joint replacement constructed from hard materials. Jin, et.al. Proc. Instn Mech. Engers, Part C. J. of Mechanical Eng Sci, 1999, 213(C4), 355.
- Analysis of fluid film lubrication in artificial hip joint replacements with surfaces of high elastic modulus. Jin, et. al. Proc. Instn Mech. Engrs, Part H, J Eng in medicine, 1997, 211 (H3), 247.
- The use of dense alumina-alumina ceramic combination in THR. Buitin, et. al. J Biomed Mater Res. 1988, 22, 1203.
- Biocompatability of surgical grade dense polycrystalline alumina. Christel, et. al. Clin Orthop Rel Res, 1992, 282.
- Bearings of the Future for Total Hip Arthroplasty. Michael T. Manley, FRSA, PhD and Kate Sutton, MA, ELS. The Journal of Arthroplasty, 2008.
- Bearings of the Future for Total Hip Arthroplasty. Michael T. Manley, FRSA, PhD and Kate Sutton, MA, ELS. The Journal of Arthroplasty, 2008.
- Ceramic Femoral head retrieval data. Willmann, et al. Con Orhtop Relat Res 2000;379
- Two-9 year clinical results of alumina CoC THA. Murphy, et al. Clin ORhtop Rel Res 2006;453
- Bearings of the Future for Total Hip Arthroplasty. Michael T. Manley, FRSA, PhD and Kate Sutton, MA, ELS. The Journal of Arthroplasty, 2008.
- Experience with the Metasul TH bearing system. Weber, et al. Coin Orhtop 1996;329(Suppl):569.
- THJ Replacement using a CoCrMo M-M sliding pairing. Weber, et al. J Japanese Orthop Assoc 67: 391, 1993.
- Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. Treacy, JBJS Br 2005;87(2):167.
- Birmingham hip arthroplasty five to eight years of prospective multicenter results. Khan, et al. J Arthroplasty 2009;24(7):1044-50
- Five-year results of MoM resurfacing arthroplasty in Asian patients. Nishii, et al. J Arthroplasty 2007;22(2):176
- The results of primary Birmingham hip resurfacings at a mean of five years. An independent prospective review of the first 230 hips. Hing, et al. JBJS Br 2007;89(11):1431.
- The five-year results of the Birmingham hip resurfacing arthroplasty: an independent series. Steffen, et. Al. JBJS Br 2008:90(4):436-41.
Related articles
- Techniques In Implementing A Hip Replacement Operation (earlsview.com)
- Minimally Invasive Total Hip Arthroplasty (earlsview.com)
- Veterans are Put through Another War – Hip Recall (earlsview.com)
- Crucial Documents Could Determine DePuy Hip Replacement Lawsuit Results (earlsview.com)
- Another Legal Victory for Victims With Recalled DePuy ASR Hip Replacement Implants (earlsview.com)
- How Long Do Hip Replacements Last? (earlsview.com)

what is suggested to those who have faulty equipment from hip surgery ?
This is a terrific overview, especially in light of N.Y. Times report this week on nationwide surge in hip-replacement complaints. Bravo! Jim Doherty, Spring Green, WI.